ATS/ERS Spirometry 2019 - At a glance

In 2019, the American Thoracic Society (ATS) and European Respiratory Society (ERS) released a long-awaited update to their international spirometry recommendations. These recommendations represented the latest in the decades-long effort to standardize the performance and interpretation of spirometry, as well as integration of advances in research and technology that have made data acquisition and analysis both smoother processes. This is especially true with cutting-edge devices like the ndd EasyOne™ line of pulmonary function testing equipment.
As benefiting a revision of its magnitude, the new technical statement is a full sixteen pages of best practices, new factors to consider, and technological standards to reach. However, there are five points that stand out above in importance and application. Recognizing that clinical time is valuable, we will highlight and summarize those key points below.
1. A full flow-volume loop is now the standard, meaning EOT (end-of-test) is now EOFE (end of forced exhalation). #
Previous versions of the recommendations focused solely on the expiratory part of the maneuver. However, adding a maximal inhalation after the maximal exhalation adds an additional quality-control measure, as the forced inspiratory vital capacity (FIVC) can be compared against the initial FVC measurement to see if they were truly maximal to start.
That change means cessation of flow no longer represents ‘end-of-test’ (EOT), but rather the end of forced exhalation (EOFE), and the standardized vocabulary has been updated accordingly. Recognizing an acceptable EOFE is critical to determining that an accurate FVC has been reached. These standards define EOFE as reaching an expiratory plateau with <25mL of change for a minimum of 1 second, reaching an overall expiratory time of 15 seconds, or a reproducible FVC (in order of preferability). Minimum forced expiration time (FET) of 6s, required by the previous standard, no longer applies according to the new standard. These new standards allow enough flexibility for the vast majority of patients to achieve an acceptable result regardless of age, coordination, disease state, or other factors.
2. FEV1 and FVC parameters are now graded for acceptability and usability. #
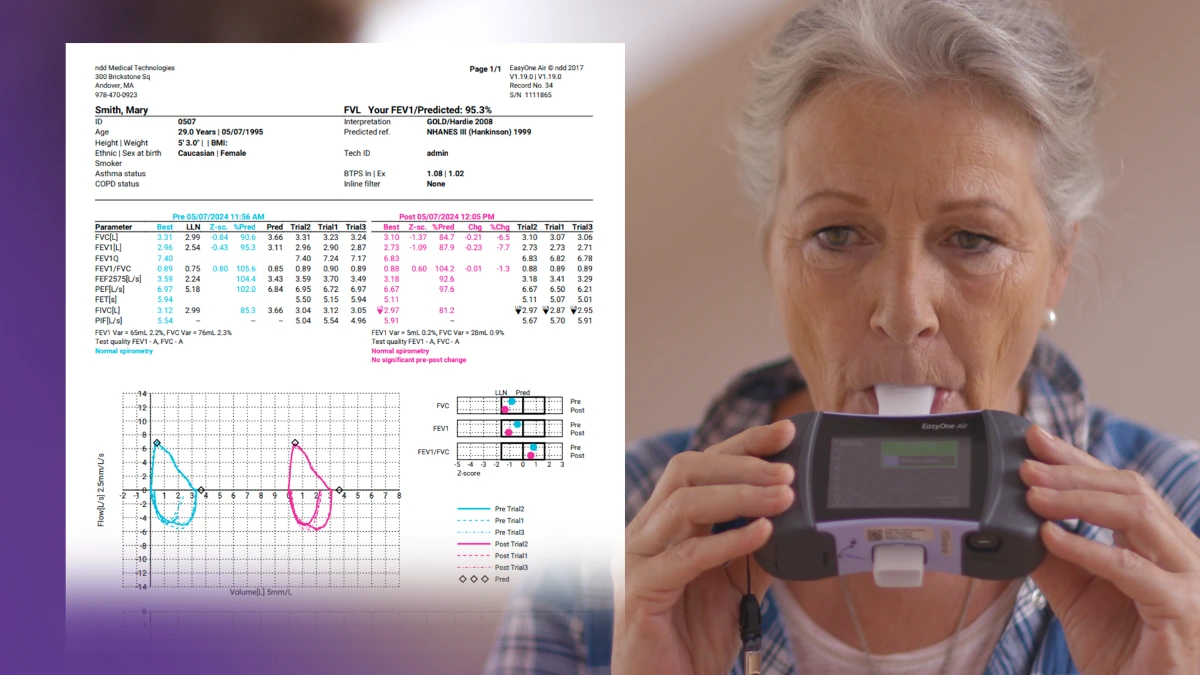
Spirometry data has long been evaluated on the basis of repeatability and alignment with quality control standards, but historically these evaluations have essentially been either pass or fail. But clinical decision-making is rarely that black or white. In recognition of this, ATS/ERS recommended the inclusion of a grading system in their 2017 technical statement on standardized pulmonary function testing reports.2 The new document expands that system, assigning a letter grade to the overall test, depending on the acceptability and repeatability of each individual measurement. Three or more acceptable efforts with FVCs and FEV1s within 150 mL of each other (100 mL in children under 6) grant a grade of ‘A’, with fewer acceptable efforts (or wider ranges of repeat values) going down all the way to ‘E.’ However, before the test is considered a complete failure and garners an ‘F,’ there is a new grade of ‘U’ (usable), representing that there was at least 1 usable measurement that may give some clinical benefit. Usability varies slightly from acceptability by allowing things like an insufficient EOFE condition, or evidence of a leak around the mouthpiece. Fortunately, these evaluations are integrated into ndd’s EasyOne Connect software, giving you immediate feedback on test quality.
3. Report parameters are clearly defined #
The 2005 iteration of the ATS/ERS standards frequently mentioned things that “should” be included in test readouts, but the new standards explicitly call for the inclusion of several datapoints. These include both the basics (FVC, FEV1, etc.) and things that may be new to this report (forced expiratory time and FIVC). In addition, reports should contain date and time, to account for the normal diurnal variation in lung function and allow appropriate tracking and trending. All data should be available in aggregate and for each individual measurement, and comments from the testing technician should be included and reported. Finally, the report calls for all information to be exportable as both a PDF and in standard electronic formats readable by electronic medical record systems. Again, all of these requirements are standard in EasyOne Connect software.
4. Changes to Slow Vital Capacity (SVC) and bronchodilator testing #
SVC measurements can be a very useful tool in evaluating emphysemic lungs, and the new recommendations provide some insight into their application. The 2019 standards call for a maximum limit of eight maneuvers (up from four), and that if the three preceding tidal breath volumes are not within 15% of each other, the inspiratory capacity measurement may not be valid or useful.
In a similar clarification, the report now describes “bronchodilator responsiveness testing,” rather than reversibility, as this was felt to imply the complete reversal of obstruction in the airways.
5. New equipment standards #
In addition to tightening the accuracy requirements from 3% to 2.5%, and the ability to add comments, the ATS/ERS report calls for additional usability features to optimize data quality. Chief among these is the recommendation that PFT equipment provide audio and visual feedback throughout each test maneuver. For example, the spirometer should provide an audible alert at the end of the forced or expiratory maneuver, or a double beep when a plateau is reached after a slow expiratory maneuver. All ndd devices meet or exceed the ATS/ERS recommendations, are designed to be interoperable with a wide range of EMR systems and networks using HL7/XML data exchange, and have been engineered for reliability and ease of use to ensure high-quality data capture and simplified interpretation at the point of care.
Time and medical research are always marching forward, and the 2019 ATS/ERS spirometry standards represent the state of the art in spirometry testing and evaluation. ndd is proud to offer a range of devices that make adhering to best practices easy and affordable for practices of any scale across the continuum of care. EasyOne devices and software empower clinicians to provide world-class diagnostic capabilities to their patients with ease. For more information on our product line, click here: