There’s light at the end of the COVID tunnel.

As more and more healthcare professionals receive their vaccine doses, it’s a bit easier to start looking ahead to how we can all deliver care after the seismic shift created by the COVID-19 pandemic. For months, most clinicians have been focusing on adapting to new protocols and procedures, as well as worrying about not only their patients’ safety, but their own as well. There’s been a great deal of talk about the so-called ‘new normal,’ but the fact of the matter is, we’ve still been in crisis mode, with very little attention paid to sustainability or proactivity.
However, that is now changing. Across the continuum, clinicians have an opportunity to not only adapt to a new normal, but actually to start creating this “normal”. Pandemic disruptions have given clinicians of every stripe a generational opportunity to rethink virtually every aspect of healthcare delivery. There is perhaps no greater opportunity to improve efficiency, positively impact population health research, and lower overall costs than in pulmonary function testing.
Don’t you need a special lab for that? #
It may seem a tall order at first. After all, traditionally, such testing has been the domain of specialized facilities with seemingly esoteric equipment. Certainly, in the early days of PFT (and even into the microprocessor age), many of the devices were relatively cumbersome. Now, however, the necessary tools are economical, far easier to operate, and can literally fit in the palm of your hand. That makes setting up a PFT program a practical option at virtually any point of care. Considering the scheduling backlogs that established diagnostic centers will be seeing for the foreseeable future (even after the decision is made to reopen safely), clinics, primary care offices, and other non-traditional locations have an opportunity to bring testing in-house, reducing the burden on patients, and potentially establishing a new revenue stream that can be sustained well into the future.
How do we get started with point of care PFT testing? #
One of the nicest features of point-of-care PFT testing is scalability. For many practices, the best place to start will be with basic spirometry testing. Spirometry is an essential, yet often overlooked, component of respiratory diagnostics; indeed, according to the American Thoracic Society (ATS), the Global Initiative for Chronic Obstructive Lung Disease (GOLD), and other professional groups, conditions like COPD cannot be accurately diagnosed without it.1 Initiating a spirometry program provides an immediate impact on care delivery (and therefore a related impact on patient satisfaction).
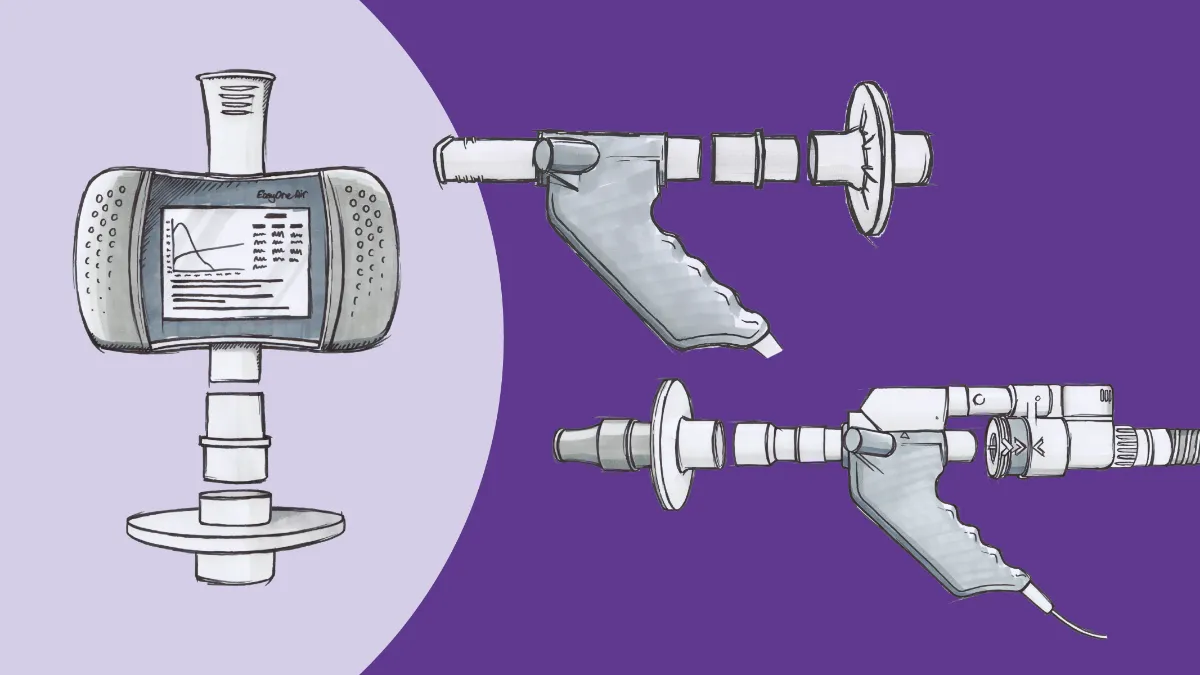
Adding the ndd EasyOne Air is a great way for any office to get their feet wet with spirometry testing. The EasyOne line has a well-deserved reputation for rugged reliability in a variety of settings. Right2Breathe, a non-profit organization dedicated to raising awareness of chronic lung disease, has relied on the device to provide preliminary test results for thousands of people at screening events held in conjunction with drag races across the country. While the clinic atmosphere is (hopefully) a little more conducive to testing than a raceway infield, many of the same features that make it a great tool for field testing also make it ideal for starter programs. Unlike most spirometers, the EasyOne Air does not require daily calibration, thanks to its unique ultrasonic TrueFlow™ measurement technology. This significantly reduces the workload of those in your clinic tasked with performing the testing. The device can be used in an entirely standalone mode (with a color touchscreen for data entry and review) or connected to a PC or laptop (via Bluetooth) to enable additional features like incentive/coaching screens, and these connectivity features allow the unit (and accompanying software) to be connected either to a printer or directly to the office EMR.
Speaking of personnel, medical practices may be concerned that adding a PFT program could require the addition of specialized staff. While bringing in a registered respiratory therapist or pulmonary function technologist is certainly optimal (and can provide additional opportunities for clinical improvement and revenue enhancement), medical practices that are just starting can still integrate POC spirometry into their existing workflows with existing staff, particularly with the EasyOne Air. An earlier generation of the unit was deployed after 9/11 by the World Trade Center Worker and Volunteer Medical Screening Program. With only some training by a pulmonologist and some practice, the technicians of this program were able to achieve ATS grade A or B tests over 80% of the time.2
POC spirometry is also easy to integrate into administrative workflows. Once the test is performed, data can be presented to attending physicians for review and sign-off (or even forwarded to a collaborating pulmonary office, depending on policy or preference). This allows for rapid evaluation of your patients’ pulmonary status and enhanced clinical decision making during the same visit. No more waiting for results from another practice or hoping your consult can even get scheduled. Reimbursement is straightforward, with two spirometry-specific billing codes (although post-bronchodilator spirometry is recommended for diagnostic purposes), and an average reimbursement of between $36-$60, per the CMS.gov Physician Fee Schedule Search. This allows for a return on investment (ROI) within approximately 60 tests. Depending on patient volume, a practice could not only enhance patient care and satisfaction, but generate additional revenue within weeks.
Finally, the EasyOne Air is designed to be infection control friendly, even in theCOVID-19 era. The same ultrasonic technology that eliminates the need for calibration also enables the use of ‘straight-through’ mouthpieces; no part of the flow sensor touches any patient-generated gas or aerosols and all testing components that do come into contact with exhaled breath are single-use disposables. The unit itself can be easily cleaned between patients with standard disinfecting wipes, and viral filters can be fitted onto the mouthpieces with simple adapters, ensuring environmental safety for patients and staff alike.
Taking point of care lung function testing to the next level #
Spirometry is far from the only game in town, though. While flow measurements remain essentially the gold standard for most respiratory diagnostics, a growing body of evidence supports the use ofdiffusion capacity (DLCO) for diagnostic and prognostic purposes, particularly for those who survive the acute phase of COVID-19. A recent study published in the European Respiratory Journal found that gas exchange problems measured by DLCO were present in 21% of COVID-19 survivors even 100 days into their recovery.3 This has important ramifications for research into “long COVID”, as well as making sure these people have access to appropriate therapeutics.
This is a test that many in primary care would feel beyond their practice’s resources or capability, but once again, modern technology jumps in. TheEasyOne Pro line brings additional testing capability to the point of care without massive investments in infrastructure or training. Admittedly, the machine doesn’t quite fit in the palm of the hand, but with a footprint of one square foot, the EasyOne Pro allows for DLCO measurements in a far more compact package than the traditional ‘body box’ plethysmograph. In addition, weighing a relatively svelte 18 pounds, the EasyOne Pro can be transported from room to room (or even clinic to clinic) with minimal effort.
The EasyOne Pro shares many of the same advantages of its smaller EasyOne Air sibling. It has the same calibration-free ultrasonic TrueFlow technology, the same user-friendly interface, and the same EMR interface capability. The DLCO test itself generally only requires a few minutes, so it can be integrated into workflows alongside spirometry testing. This low administrative overhead means that the clinical return with this investment can be even more valuable; not only is DLCO a clinically relevant indicator in long COVID, but impaired DLCO has also been associated with higher morbidity and mortality in COPD4, hospitalization secondary to COPD5, and mortality in heart failure with reduced ejection fraction.6 That last one might be a surprise, but it’s important to remember the heart and lungs are neighbors in every sense of the word, and what affects one usually affects the other, making it even more important to have a full range of testing available.
DLCO testing provides another potential revenue source as well. The average national reimbursement for the test, according to CMS.gov, is approximately $57. That may not sound like a lot for a more advanced test but remember that’s in addition to any spirometry done during that visit, and the EasyOne Pro can do both tests during the same session. Add in another $43 for lung volume testing (extremely helpful for measuring the degree of air trapping often present in people with COPD), and practices can achieve a full return on investment in as little as three months simply by averaging one test series per workday. No massive recruitment push, no deep dives into patient records to scrape as many people as possible – just one person with shortness of breath, heart problems, or chronic cough each day. With over 16 million people in the US diagnosed with COPD, another 16 million walking around undiagnosed but with symptoms, and millions more with various kinds of cardiac problems, it’s a rare clinic that can’t find that daily tester.
While the EasyOne Pro does have some additional technological and administrative burdens compared to the EasyOne Air, ndd has tremendous experience in making the process as simple as possible. The ‘CO’ in ‘DLCO’ stands for carbon monoxide, which is (hopefully) not something one finds in the average clinic supply closet. However, ndd’s customer support team can easily connect clinics with local gas wholesalers, ensuring a smooth process from the start. Like their smaller sibling, neither the EasyOne Pro nor the EasyOne Pro LAB require daily calibration; and along with TrueFlow, the EasyOne Pro uses proprietary TrueCheck™ technology to validate each and every test, without the need for simulators or other external controls. What maintenance these machines do need can be done by a single person in approximately ten minutes on an annual basis.
Pulmonary function testing for the masses #
There are thought to be roughly 5,000 pulmonary function laboratories scattered across the United States, with most of them attached to or near a hospital7. All of them are currently struggling with service interruptions, scheduling backlogs, and all of the other issues created by the pandemic, limiting their ability to provide timely diagnostic services and care coordination with patients, caregivers, and other healthcare professionals. Even before COVID-19, there were simply not enough labs to go around, and adding another stop to patients’ clinical routes led to them being ‘lost to follow up.’ Despite the dawn of 2021, those issues are unlikely to go away anytime soon. This ‘diagnosis gap’ leads to delays in treatment and quality of life improvements for a variety of chronic cardiorespiratory conditions.
That’s where the over 100,000 primary care practices around the country (according to market research by IQVIA) enter the picture. With cost-effective, user-friendly solutions now available, it’s easier than ever for non-pulmonary practices to add pulmonary testing to their repertoire. A future where routine ambulatory checkups include not only old favorites like heart rate and blood pressure, but key indicators like FEV1 and DLCO is not only foreseeable but within reach. Adding pulmonary function testing is an inexpensive way for any practice to have a huge impact on their patients, their communities, and the overall healthcare system, ensuring that the light we all see at the COVID tunnel shines as brightly as possible for everyone.
Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-191. doi:10.1059/0003-4819-155-3-201108020-00008 GOLD. The Global Initiative for Chronic Obstructive Lung Disease (2019). Global Strategy for the Diagnosis, Management and Prevention of COPD. Retrieved from. Gold. 2019. ↩︎
Enright PL, Skloot GS, Cox-Ganser JM, Udasin IG, Herbert R. Quality of spirometry performed by 13,599 participants in the World Trade Center Worker and Volunteer Medical Screening Program. Respir Care. 2010;55(3):303-309. ↩︎
Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur Respir J. December 2020. doi:10.1183/13993003.03481-2020 ↩︎
Balasubramanian A, MacIntyre NR, Henderson RJ, et al. Diffusing Capacity of Carbon Monoxide in Assessment of COPD. Chest. 2019;156(6):1111-1119. doi:10.1016/j.chest.2019.06.035 ↩︎
Wei X, Ma Z, Yu N, et al. Risk factors predict frequent hospitalization in patients with acute exacerbation of COPD. Int J COPD. 2018;13:121-129. doi:10.2147/COPD.S152826 ↩︎
Magnussen H, Canepa M, Zambito PE, Brusasco V, Meinertz T, Rosenkranz S. What can we learn from pulmonary function testing in heart failure? Eur J Heart Fail. 2017;19(10):1222-1229. doi:10.1002/ejhf.946 ↩︎
Kaminsky DA, McIntyre N, Culver B. The pulmonary function laboratory: Something old and something new. Ann Am Thorac Soc. 2017;14(1):10-11. doi:10.1513/AnnalsATS.201610-763ED ↩︎