Why ndd's TrueFlow Technology is leading the way in spirometry and pulmonary function testing

Pulmonary function tests (PFTs) are essential medical tests used to diagnose conditions like asthma and chronic obstructive pulmonary disease (COPD). But their role does not stop at diagnosis. PFTs are also important for tracking the progression of lung diseases and evaluating how well patients respond to treatments, including medications tested in clinical trials.
Asthma and COPD are often underdiagnosed, which highlights the need for greater accessibility to diagnostic tools. By improving access to tests like PFTs, we can close the gap and ensure more people receive proper diagnosis and treatment, earlier.
Understanding TrueFlow Technology #
Most PFT devices have mechanical parts, including a turbine, to assess the volume of air someone exhales during their test. Mechanical parts also mean more parts in general and therefore can involve more potential points of failure. Usually that also means they need to be calibrated, repaired, and troubleshooted more often.
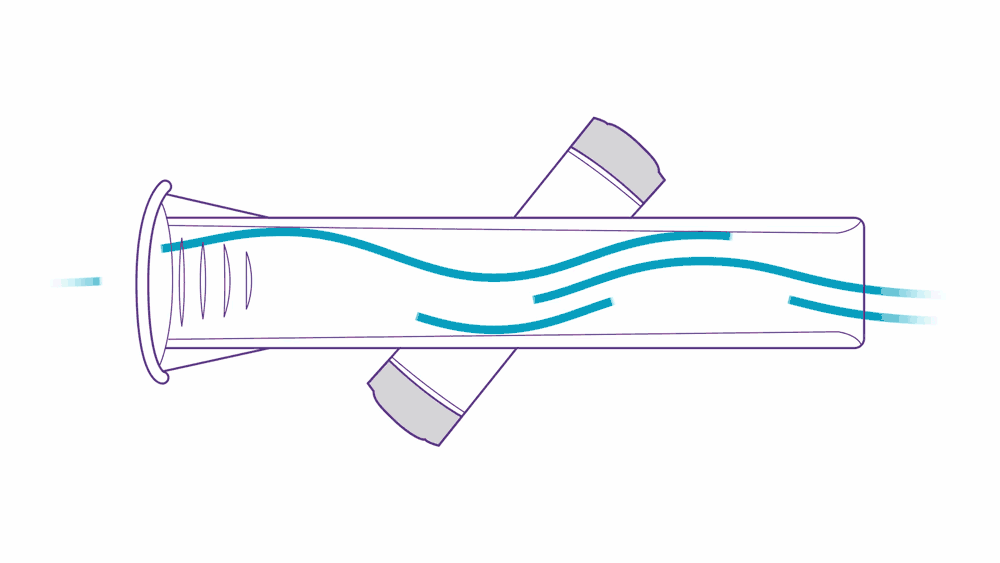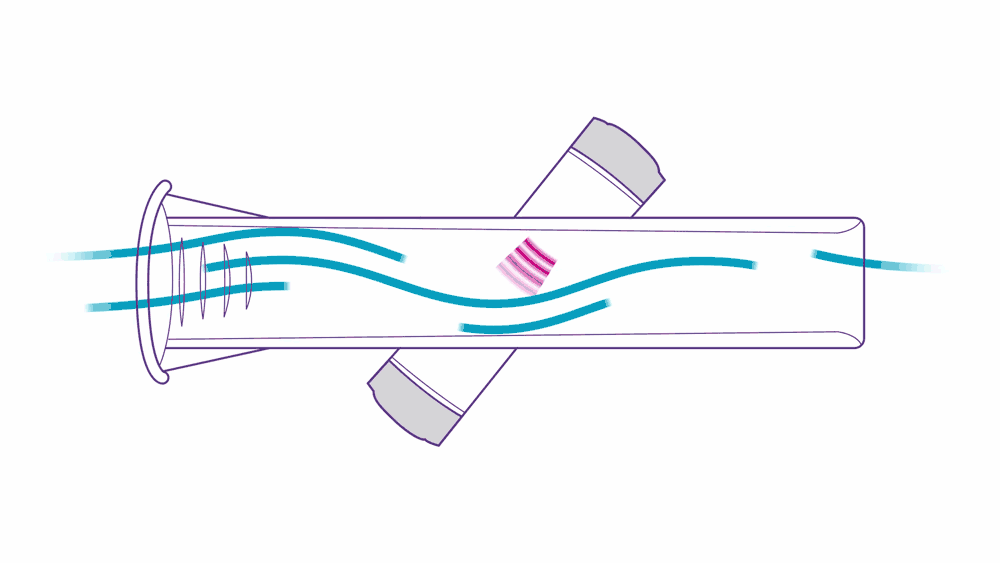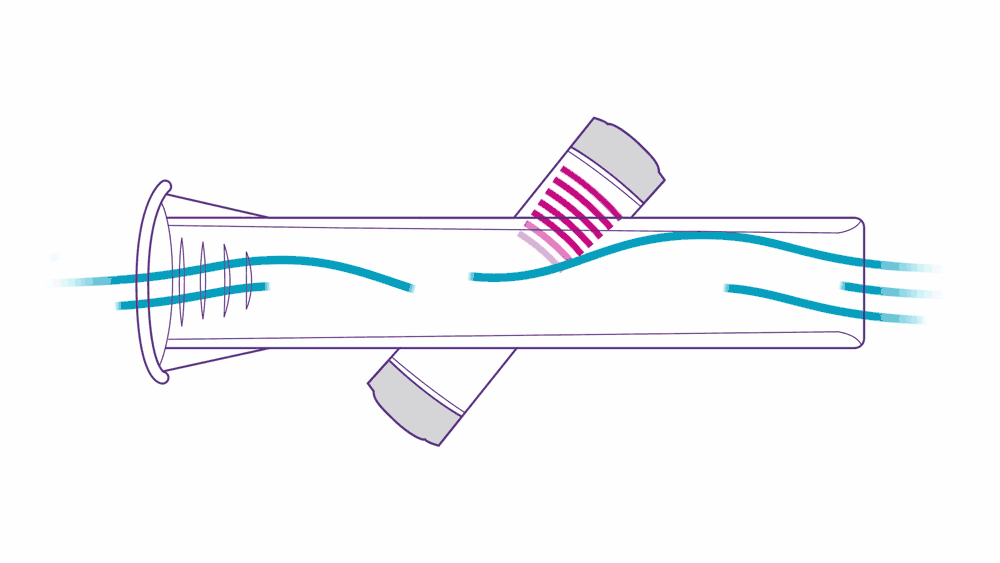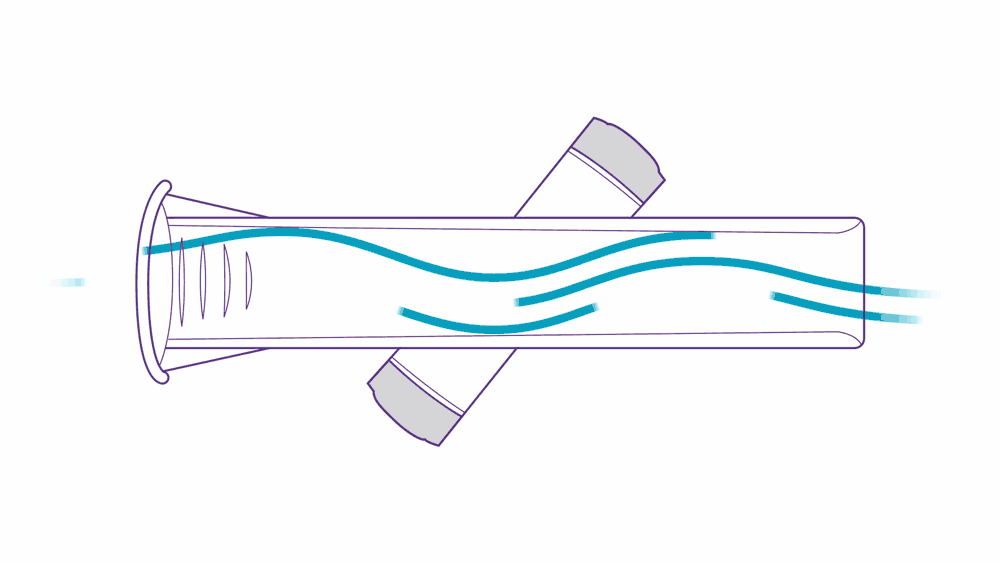
Ultrasound PFT devices leverage ultrasonic pulse transit-time measurement.
Here’s how it works: two transducers positioned diagonally pulse high frequency sounds toward one another. As a patient performs their test, they exhale air into the tube, which accelerates the upstream pulse and slows down the downstream pulse — this is what “transit-time measurement” means, and it’s a simple, precise, and proven method of measurement.
This approach to assessing flow volume is not only accurate, but also highly reliable. ndd breathing mouthpieces are precisely engineered to fit ndd devices, providing accurate, low-resistance measurements. They help prevent cross-contamination and reduce the need for device cleaning.
Key benefits of TrueFlow Technology #
- Calibration-free - TrueFlow technology eliminates the need for regular calibration, leading to consistent accuracy and reducing staff efforts on regular calibration and troubleshooting.12
- High durability and longevity - ndd Medical Technologies’ EasyOne devices are durable and stable over the device’s lifespan,3 offering a high return of investment for clinics.
- Measurement accuracy - EasyOne devices are precise at both high and low flow rates, a crucial consideration for detecting early-stage respiratory conditions.
- Versatile application across diverse settings - TrueFlow technology enables EasyOne devices to deliver precise results across a wide range of settings—from pediatric to adult care, rural community hospitals, and even extreme environments. Its accuracy has been proven in diverse conditions, including research settings from the high altitudes of Mount Everest to the shores of The Bahamas, where it has been used to assess freedivers.*
Broad spectrum of application #
The reliability, simplicity, and accuracy of EasyOne devices, powered by TrueFlow technology, have made them highly regarded for clinical and veterinary research studies, where maintaining high standards and reliability is crucial.*
EasyOne devices have been used as part of major international clinical studies:
- The BOLD4 and PLATINO5 Studies aiming to understand the global burden of COPD.
- The World Trade Center Study that sought to understand the long-term effects of exposure to dust that thousands of New York City firefighters experienced after the 9/11 attacks.
- The COPDGene Study that investigated the genetic underpinnings of COPD.6
- And the Multicenter AIDS Cohort Study which is a 30-year-long study researching the impact of HIV on pulmonary function.
Additionally, TrueFlow technology has been used in veterinary research studies to better understand marine animals too:
- Study and treat endangered sea turtles trapped in fishing nets
- Research lung function and pulmonary disease in dolphins and whales
EasyOne spirometry machines - tailored solutions for every practice.
Explore our spirometry machines
Conclusion #
ndd’s TrueFlow is innovative in its ingenuity, brilliant in its simplicity, and a breakthrough in how it addresses common challenges in pulmonary function testing. This technology offers a reliable, accurate, and user-friendly solution.
Healthcare providers around the world – whether it be primary care, pulmonology or cardiology, community or rural – can benefit from leveraging EasyOne devices in their clinics.
Explore ndd’s spirometry and PFT product line and experience the benefits firsthand.
* EasyOne products are medical devices and intended for use in a healthcare setting by clinicians. Some examples discussed in this blog for research purposes only.
Hegewald MJ, Gallo HM, Wilson EL. Accuracy and Quality of Spirometry in Primary Care Offices. Ann Am Thorac Soc. 2016;13(12):2119-2124. doi:10.1513/AnnalsATS.201605-418OC ↩︎
Skloot GS, Edwards NT, Enright PL. Four-year calibration stability of the EasyOne portable spirometer. Respir Care. 2010;55(7):873-877. ↩︎
Pérez-Padilla R, Vázquez-García JC, Márquez MN, et al. The long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care. 2006;51(10):1167-1171. ↩︎
Amaral AFS, Potts J, Knox-Brown B, et al. Cohort Profile: Burden of Obstructive Lung Disease (BOLD) study. Int J Epidemiol. 2023;52(6):e364-e373. doi:10.1093/ije/dyad146 ↩︎
Menezes AM, Victora CG, Perez-Padilla R. The Platino project: methodology of a multicenter prevalence survey of chronic obstructive pulmonary disease in major Latin American cities. BMC Med Res Methodol. 2004;4:15. doi:10.1186/1471-2288-4-15 ↩︎
Regan EA, Hokanson JE, Murphy JR, et al. Genetic Epidemiology of COPD (COPDGene) Study Design. COPD. 2010;7(1):32-43. doi:10.3109/15412550903499522 ↩︎
Written by

Tré LaRosa
Tré LaRosa is a consultant, scientist, and writer in the Washington, DC area with extensive experience working in research (basic, translational, and clinical) and on patient-reported outcomes. He has also written extensively on neuroscience, pulmonology, and respiratory conditions, including from the patient perspective. He enjoys learning, reading, writing, spending time outdoors, and telling everybody about his mini golden retriever, Duncan.