EasyOne Pro LAB

Patient and User Centered Design
The EasyOne Pro LAB is both patient-friendly and easy to maintain. For MBW you only need an oxygen supply, no expensive SF6 gas required. Medical professionals can perform annual maintenance themselves in only 10 minutes.Calibration-free TrueFlow™ Technology
Extremely robust and accurate flow results are delivered without calibration thanks to our TrueFlow™ technology integrated into the EasyOne Pro LAB.Self-Quality Control through TrueCheck™ Technology
With EasyOne Pro LAB you can fully focus on the patient. TrueCheck™ ensures that each gas measurement is accurate without calibration and warm-up time.Compact & Lightweight Design
Weighing only 18 lbs. (9kg), the EasyOne Pro LAB is compact & durable – without compromising quality. It easily fits in any office setting.
Why our customers love us
We are over-the-top satisfied with ndd’s EasyOne Pro.
The EasyOne Pro is easy to use, easy to assemble, and is great for large and claustrophobic patients. The EasyOne Pro saves a huge amount of floor space, and it’s portable.
We’ve had many patients say they will never go back to the surrounding hospitals because of the convenience of testing with ndd’s EasyOne Pro (and the fact that they don’t have to enter that uncomfortable PFT body box).
- The pulmonologist that tests at our facility is impressed with the EasyOne Pro, as the testing results are spot-on with a similar PFT test done in a traditional body box. We would go with the Pro over a traditional body box any day.
- We recently acquired several EasyOne Pros for three hospitals in Madrid. Given the current situation [COVID-19], this equipment guarantees proper cleaning & disinfection between patients. In addition, it’s very easy to use, something the Team very much appreciates - it’s portable, and it brings the highest accuracy. An excellent medical device.
- Being on a truck we really like equipment that can be mobilized. It has to be small, it has to be able to be moved around without breaking, it has to be reliable. When I did the research, the EasyOne Pro was being used in that capacity for other outreach clinics and I thought we should invest in that for our trucks. It has been a great decision actually. It’s very easy to use, reliable and accurate.
- We see that ndd technology is always accurate when comparing it to simulator data or biological control values. In addition to the outstanding accuracy the ndd software is very user-friendly, which makes ndd devices easy to use.
- My clinical research on asthma and COPD happens at the point of care in some of the most remote, resource limited places in the world. Because of this, I need highly portable, very durable diagnostic devices reporting consistent and accurate results even under the toughest conditions. I use ndd’s devices because I know no matter the condition, the device will report accurate results.
- Our academic activities related to trainings and continuous learning for the specialists that take care of the respiratory health in our region, are supported by new technologies. Our experience with ndd devices has been very positive because they adapt to our necessities, they are always updated regarding new standardization and technological advances.
- The EasyOne Pro’s portability and ability to accurately perform mobile PFTs in under 30 minutes make it the perfect device for this type of clinic. It allows us to use the patients’ time more efficiently, better understand their illness, and improve assessment of their condition. It’s easy to operate and allows us to provide prompt and accurate assessment of patients’ lung function.
Testing at the Point of Care
Get maximum efficiency and patient comfort. TrueFlow™ and TrueCheck™ ensure optimal results, independent of the clinician.
- Always safe & ready to test with TrueCheck™
- Diffusion capacity results in 3 minutes
- User- and patient-friendly design
- Ideal also for claustrophobic and obese patients
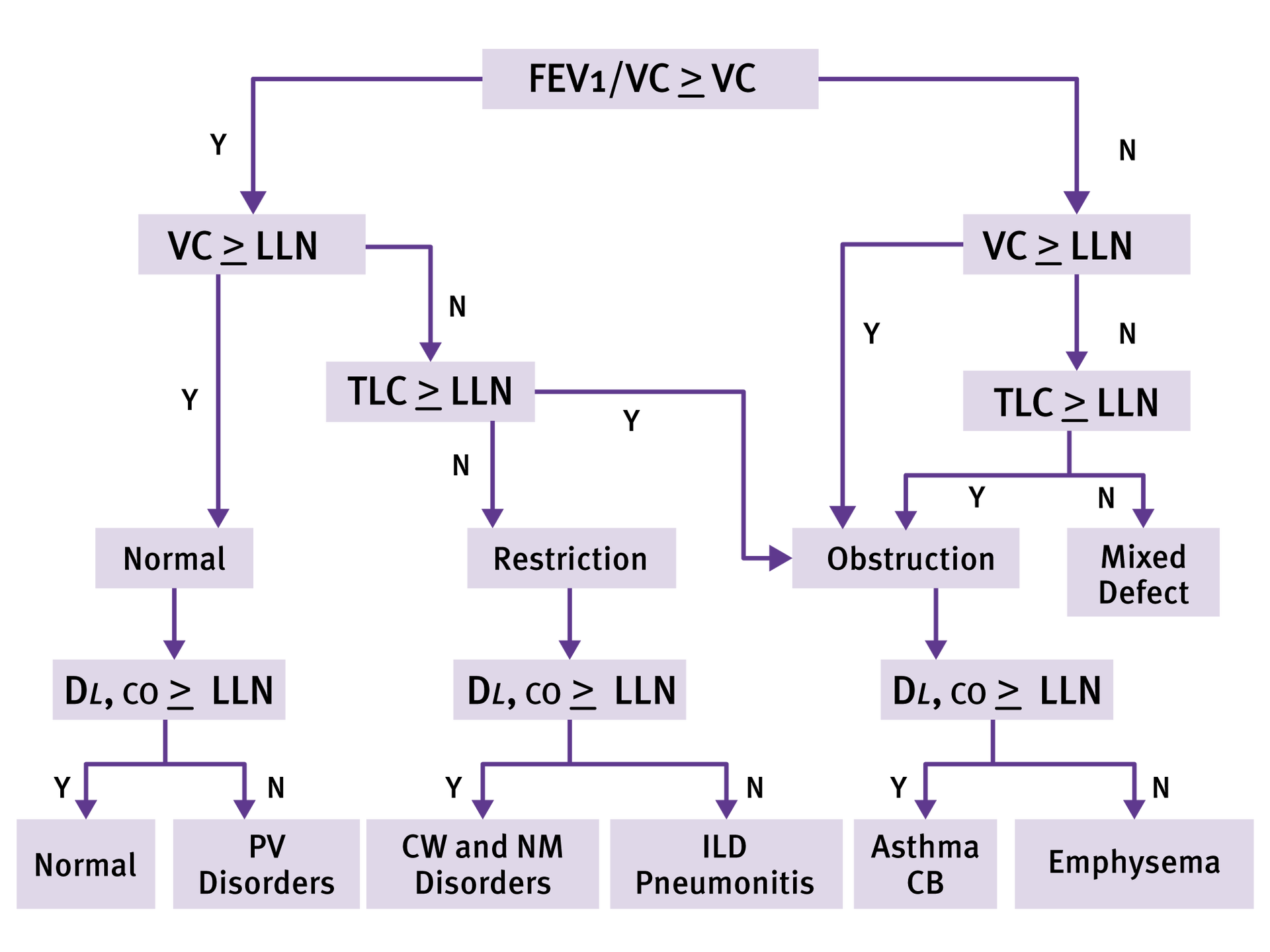
QC-Grading and Result Interpretation
Get automatic quality grading and interpretation based on NLHEP and ATS/ERS with the EasyOne Pro.
- Quality grades based on NLHEP and ATS/ERS 2005 and 2019 for all tests
- ATS/ERS interpretation decision tree
- GOLD/Hardie, NICE, JRS and NLHEP
- Diagnose Asthma, COPD, Emphysema and pulmonary vascular disorders
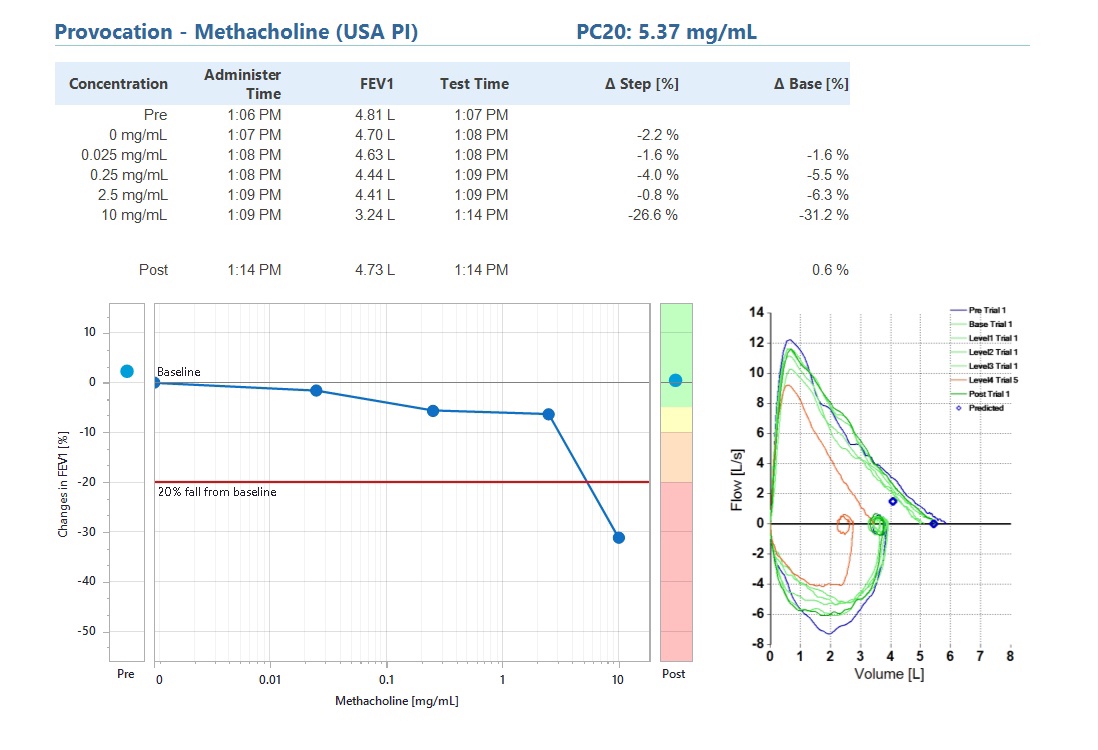
Custom Provocation Protocols
Customize available standard provocation protocols for your needs or create your own. You can easily install the customized provocation protocol on your ndd devices.
- Build your own provocation protocols
- Modify existing provocation protocols
- Export and load protocols onto other devices
Pre-installed protocols: Mannitol, MC, Exercise Provocation
Watch our video guide for more details.
TrueCheck™ – Always Safe & Ready to Test
TrueCheck™ takes care of the essential quality control for gas analysis testing. EasyOne Pro is the only device proven to be accurate for a lifetime for DLCO measurements.
- Calibration-free with proven long-term stability
- Checks gas-sensor linearity and delay
- Checks for leaks and gas concentration
- Patented technology
To review case studies and additional information about our proven technology, please click here.
TrueFlow™ - Trusted Ultrasound Technology
Stop worrying about calibration or accuracy of flow measurements. TrueFlow™ is the only ultrasound technology proven to be accurate for a lifetime for flow and volume measurements.
- Proven long-term stability
- Contact and resistance-free measurement
- Excellent accuracy and robustness
- Patented technology
To review case studies and additional information about our proven technology, please click here.
Infection Control
ndd inline filter solutions provide additional COVID-19 protection for those who wish to include a filter when performing tests.
- The filter keeps the ambient environment clean for technicians and patients
- The Spirette protects the flow sensor from contamination
- Fully passes 24 Waveform tests as required by the ATS/ERS standard
Avoid cross-contamination and reduce cleaning to an absolute minimum:
- All parts exposed to the patient’s breath are single-patient-use
- Sensor is protected from contamination by ndd breathing mouthpiece
- Only simple surface cleaning required of the device
- No special storage conditions required for single-patient use Spirettes and adapters

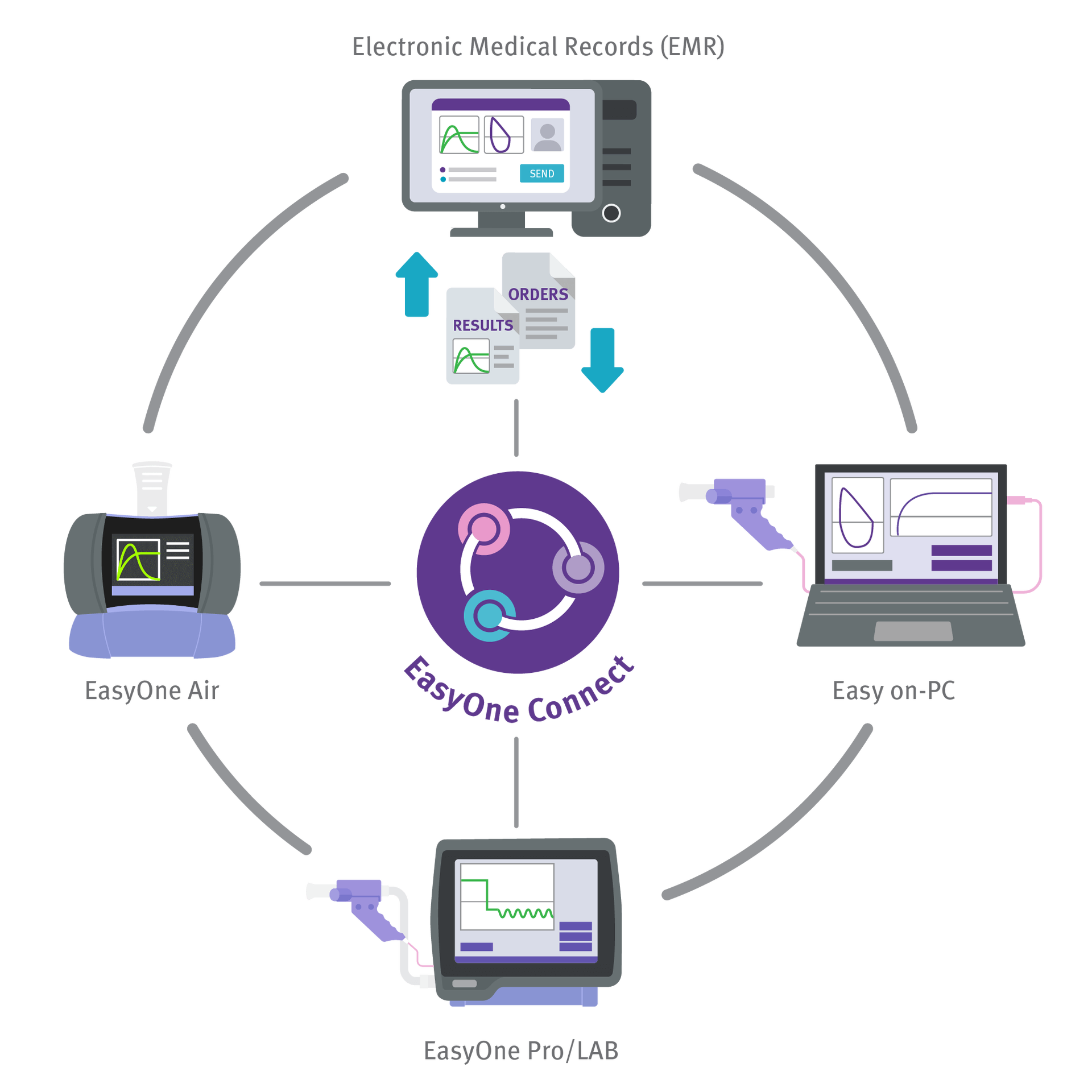
EMR/EHR Connectivity and Centralized Datav
Transmit and share data among ndd devices or to your Electronic Medical Record system.
- Integrated into all major EMR/EHR systems via HL7, XML, GDT or API
- References for integration available on request
- Simple local or SQL server database
- Review test results from any PC using EasyOne Connect
Printer and Network Connectivity
Stay connected and easily share data or print reports. EasyOne Pro is equipped with a modern embedded PC solution that makes network and printer connections extremely easy and stable.
- USB / Network printer compatibility
- Easily integrate into any network
- USB, HDMI, Ethernet
- Optional WiFi dongle

Cyber Security Protection for IT and Patient Information
Protect your patient information. EasyOne Pro is a modern system designed to fulfill your cyber security needs.
- Windows Defender Antivirus and Firewall
- Operating system updates
- Encrypted database export
- User authentication with automatic log off
- FDA Approval in 2017 with cyber security documentation
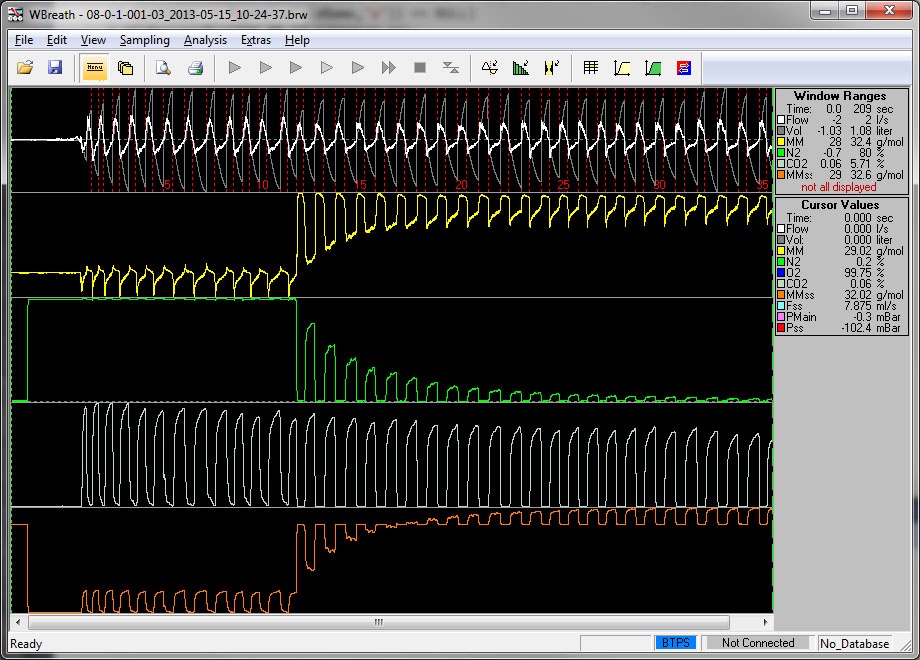
Analysis and Recording of Raw Sensor Data
Go beyond normal lung function testing and get access to all raw data of any test collected. The tool WBreath is used in special study setups, but also by our support team.
- Access and measure raw data
- Advanced Data Analysis
- Fully integrated
Worldwide Compliance with Standards
Stay compliant with ATS/ERS standards and specific country regulations.
- Full compliance with ATS/ERS 2005 and 2019 and relevant ISO standards
- Registrations across the globe
- CE Approval
- US FDA cleared to market

Downloads
 Software
Software

- EasyOne Pro V5 Updater V03.09.07.05.zipRelease date: 2025-09-01Related documents
Software is specific to your device version. Please verify before updating. Serial number range (Pro 55xxxx, Pro SN 56xxxx, LAB 65xxxx, LAB SN 66xxxx)
USB storage key needs to be min 5 GB and in exFAT or NTFS format.
 Application Notes
Application Notes

 Brochures
Brochures

 Certificates
Certificates

 Manuals
Manuals

FAQs
Which EHR vendors can ndd integrate with?
ndd can integrate with any EHR vendor that can support a bi-directional HL7 orders/results lab interface.
What kind of integration options does ndd offer?
ndd offers a file-based integration utilizing an SFTP or network-based location for file communication with an EHR vendor. HL7 is the standard data structure that ndd utilizes but can support other data formats as well. The use of other interface engines can be utilized to complete an integration project.
What is the process for integrating your software with our existing systems?
The first step for an integration project is to complete the EMR integration request form here: https://nddmed.com/f/emr. An integration specialist will then reach out to you with the next steps.
How long does the integration process typically take?
Duration depends on the IT team of the facility. The ndd side of the integration process can be completed within a few hours for standard integrations. EasyOne Connect software is simply configured to point to the SFTP or network-based location where files are being placed.
How does ndd ensure that data is securely transferred during integration?
EasyOne Connect software utilizes AES 256 encryption. SFTP has its own security protocols built into it. If a network-based location is utilized, it will follow the protocols of the facility.
Can patient data and test results be accessed across multiple laptops and desktops within a clinic?
Yes, EasyOne Connect offers an optional centralized patient database feature, enabling seamless access to patient orders and results across all instances of the software, irrespective of physical location. Customers can leverage their on-premise SQL servers or MS Azure environments to ensure data accessibility and consistency.
Does ndd offer user management within the software?
EasyOne Connect provides flexible options for managing users and their access. User accounts can be created and managed directly within EasyOne Connect for simple permission control. For more advanced management, the software supports integration with Active Directory for using your existing directory service accounts, groups, and permissions. EasyOne Connect also supports optional single sign-on (SSO) capability. If you would like to review the Active Directory Application Note, please contact [email protected].
Can ndd provide examples of successful integration with other clients?
ndd has integrated with all major vendors and can provide examples of successful integrations with various healthcare providers and organizations. You can contact our integration department via email at [email protected] for more information.
How does ndd handle updates and maintenance to the integration?
ndd does not handle the maintenance and upgrades as the software is installed locally on the client’s machine. We do, however, offer a silent installer package that our support team can assist you with. This silent installer can be executed via a script through active directory commands depending on your facility. It is the responsibility of the facility to implement any future updates. There is no licensing fee associated with the software and/or the updates.
Are there any additional costs associated with integration?
ndd does not charge for standard integration. In the event custom development is needed, ndd will provide a statement of work before a project begins. Almost all EHR vendors will charge a fee that the client is responsible for.
What are the HL7 specifications?
HL7 specifications can be found here.
What are the database options?
- Local SQLite file-based database.
- On-premise Microsoft SQL Server database.
- Azure cloud-hosted Microsoft SQL Server database.
Please see the application note for additional information.
Who is responsible for setting up and hosting the SFTP and/or network-based location?
The facility is responsible for setting up and hosting either option. ndd requires a username and password if using an SFTP environment.
What results are received in the HL7 message?
The result HL7 message will consist of a combination of discrete results and an embedded base64 PDF attachment for the PFT report. If you prefer, we also offer the option of including a reference link instead of the embedded PDF.
Is a server required?
No, a server is not required.
What kind of support does ndd offer during and after the integration process?
You can schedule a meeting with an integration engineer from 8 a.m. to 5 p.m. ET Monday through Friday. ndd can accommodate meetings outside of that timeframe if planned accordingly. The integration team can be reached via email at [email protected]. The support team can also be reached via email at [email protected] or by phone at (978) 470-0923.
What is the difference between the EasyOne Pro and Pro LAB?
The EasyOne Pro LAB is exactly the same as the EasyOne Pro, except that it has the capability to perform the multi-breath washout (MBW) test. This test requires additional sensors and the use of 100% Oxygen.
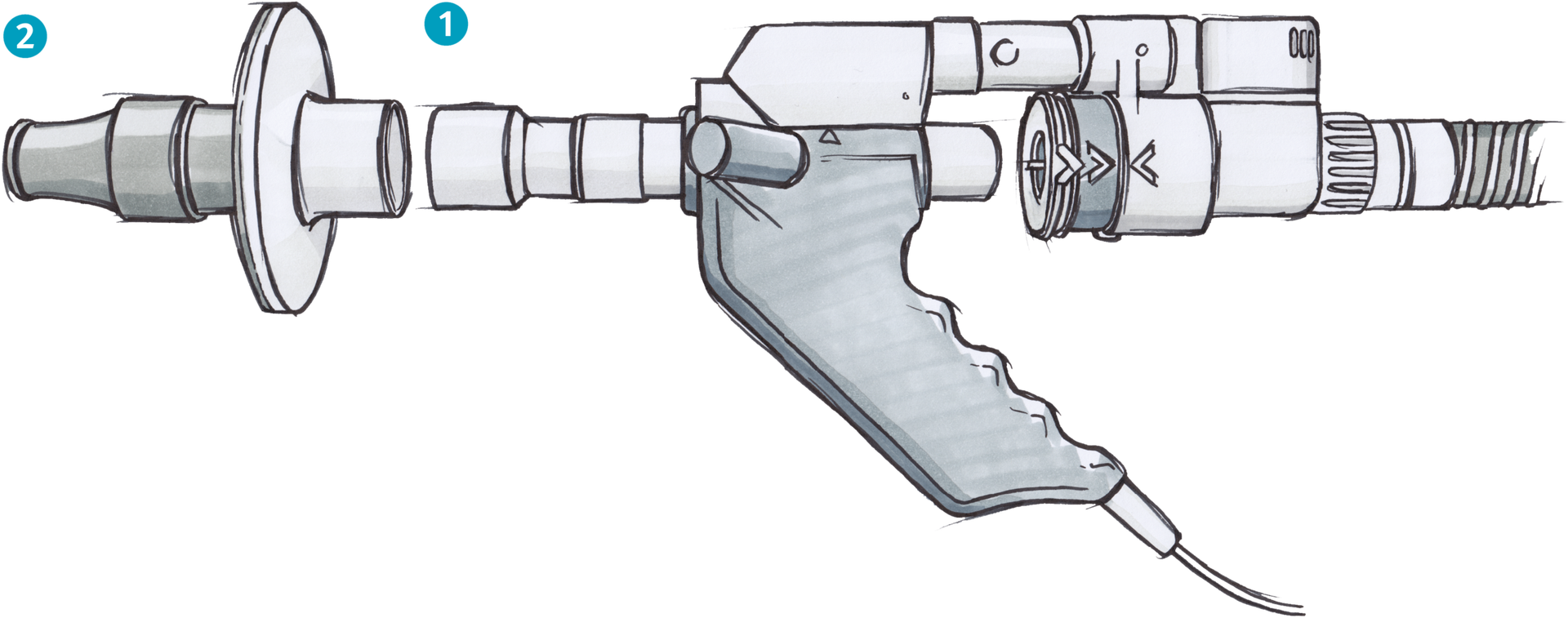
How to connect a filter to the EasyOne Pro/LAB
To add a filter (2) to the EasyOne Pro, insert the (1) Spirette FA Pro/LAB into the EasyOne Pro handle. Then attach the filter to the Spirette FA Pro/LAB, as shown in the diagram below. If you have questions about how to connect or use ndd filters, please contact ndd customer support in North America at [email protected] and internationally at [email protected].
Which predicted or normal values are supported?
You can find the product specification on the respective product pages. The specification contains a list of all supported normal values.
To get more detailed information about the supported parameters, age range, ethnicities and height range of each supported predicted set, please click here.
How do you derive DLadj (DLCO adjustment)?
The DLCO value can be adjusted for hemoglobin, altitude and the COHb concentration and CO back pressure.
Click here to find more information about the formulas used to derive DLadj.
How can I perform Phase-III Slope Analysis including Scond and Sacin?
Our research software WBreath can be used to perform a slope analysis. Please click here to get more information on this topic.
What is BTPS and how is it used?
BTPS Correction is used to convert flow and volume measured at ambient conditions to the conditions within the lungs. Ambient conditions are called ATP (ambient temperature, pressure); the conditions within the lungs are called BTPS (body temperature, pressure, water vapor saturated).
Click here for more information.
Can the EasyOne Pro/LAB be connected to my network for backup and data sharing?
Yes. Connecting the device to your network allows several additional benefits.
o Take advantage of your network data backup processes
o Print directly to your network printers
o Save reports electronically (such as PDF) directly to a network folder
o Connect with your EMR system
o Take advantage of a more powerful SQL Server database engine (click herefor more detailled instructions)
o Connect multiple devices to a single, shared database
o Get access to the device database from your PC using our free EasyOne Connect PC software application
Do I need any special calibration equipment for the EasyOne Pro/LAB?
All EasyOne products are designed to require no calibration and minimal maintenance. The device is designed to remain in calibration throughout its lifetime. No calibration procedures are required prior to testing. Our TrueFlow ultrasonic technology assures accurate volume and flow measurements every time. And our unique automated TrueCheck process assures that all gas measurements are accurate for each test. You may choose to use the optional 3-liter calibration syringe to verify volume and flow accuracy, as recommended by ATS/ERS.
How long does it take to test a patient with the EasyOne Pro/LAB?
A PFT session including spirometry and DLCO generally takes less than 20 minutes.
What are my options for printing results from the EasyOne Pro/LAB?
The EasyOne Pro/LAB is compatible with virtually all PC-style USB printers, without the need for external driver installation. The printer can be connected directly to a USB port on the rear of the device. We can sell you a printer with the device, or you can supply your own.
You can also connect the device to your network where it will automatically find any connected, compatible printers avoiding the need for a dedicated printer for your PFTs.
What consumables are required for the EasyOne Pro/LAB?
Spirette: Each test performed requires the use of a spirette. This is a single-patient-use mouthpiece which ensures that no contamination can reach the internal flow path of the device. This means that no cleaning is required between patients, and no calibration, ever. Simply toss the spirette after each test session.
Barriette: For a DLCO test, in addition to the spirette, you will need to use a barriette. This protects the valve and hose system from contamination, eliminating the need for cleaning between patients.
FRC barriette: This is similar to the DLCO barriette, but used only when performing a multi-breath washout test.
Gasses: The DLCO test requires a standard medical grade DLCO gas mixture consisting of 0.3% Carbon Monoxide, 10% Helium, 21% Oxygen, remainder Nitrogen. The multi-breath washout test requires 100% Oxygen. These gasses are readily available from your local medical gas supplier in various tank sizes. We will assist you in selecting the appropriate tank regulators required for connection to the device.
How much space will an EasyOne Pro/LAB require in my office?
The device itself is self-contained and measures only 270 x 335 x 270 mm (10.6 x 13.2 x 10.6 inches) (h x w x d). No additional PC, monitor, keyboard or control unit is required. This allows it to easily fit on a counter. You will also need to allow for storage of the gas tank(s) which come in a variety of sizes, and can usually fit comfortably in a corner, or under the counter.
What are the maintenance requirements for the EasyOne Pro/LAB?
The EasyOne Pro/LAB is designed to require minimal maintenance. The annual replacement of four external components is ALL that is required to keep the system running smoothly. These components are available as a convenient kit. Installation is done in minutes and requires no special tools.
How do I backup my patient data?
Data can safely be exported by using the “Export Data” feature or “Backup” feature within the Storage tab in the configuration.
What are examples of obstructive lung diseases?
A few examples of obstructive lung disease include:
- Chronic Obstructive Pulmonary Disease (COPD)
- Emphysema
- Chronic Bronchitis
- Asthma
- Bronchiectasis
What are examples of restrictive lung disease?
A restrictive lung disease is exactly that – one where a restriction occurs in the respiratory system.
A few examples of restrictive lung disease include:
- Interstitial lung diseases such as idiopathic pulmonary fibrosis
- Sarcoidosis
- Obesity
- Scoliosis
- Neuromuscular diseases such as muscular dystrophy or ALS
What are the disease states that are associated with high Diffusion Capacity (DLCO)?
- Asthma
- Left-to-right intracardiac shunts
- Polycythemia
- Pulmonary hemorrhage
Understanding lung function tests and their importance in respiratory health.
Read the blog
What are the disease states that are associated with low Diffusion Capacity (DLCO)?
- Emphysema
- Interstitial Lung Disease
- Idiopathic Pulmonary Fibrosis
- Cystic Fibrosis
- Congestive Heart Failure
- Primary Pulmonary Hypertension
- Chronic Pulmonary Emboli
- Anemia
- Left-sided heart disease (systolic and diastolic heart failure, Mitral Valve Disease)
Understanding lung function tests and their importance in respiratory health.
Read the blog
What is a complete pulmonary function test?
A complete pulmonary function test is a comprehensive assessment of lung function that includes three key components: spirometry (possibly pre/post bronchodilator), lung volume measurements, and diffusing capacity for carbon monoxide (DLCO).
Together, these tests provide a full picture of how well the lungs are ventilating (moving air), how much air they can hold, and how efficiently they transfer gases like oxygen into the bloodstream.
Explore how ndd’s EasyOne solutions bring accuracy and ease to every step of pulmonary function testing.
Learn more
What is a pulmonary function test?
A pulmonary function test (PFTs)are a group of noninvasive tests that monitor the performance of the lungs. They measure lung volume, capacity, rates of flow, and gas exchange.
Adding the following: PFTs typically include several different types of tests:
- Spirometry, which measures how much air you can breathe in and out and how quickly you can exhale.
- Lung volume measurement, which determines the total amount of air the lungs can hold.
- Diffusion capacity test (DLCO), which evaluates how efficiently gases like oxygen move from the lungs into the blood.
Together, these tests provide a comprehensive picture of lung function and can be used to monitor lung performance over time or assess the impact of environmental or occupational exposures.
EasyOne complete PFT and spirometry solutions
Discover the EasyOne solutions
What is Diffusion Capacity (DLCO) testing?
Diffusion capacity (DLCO) is a clinically useful test that provides a quantitative measure of gas transfer from the lungs to the blood. It complements spirometry in evaluating and managing patients with cardiac and/or respiratory disease.
Why DLCO is important in lung disease diagnosis and management:
- Helps differentiate between obstructive and restrictive lung patterns.
- Detects early changes in gas exchange, even when spirometry appears normal.
- Assists in evaluating interstitial lung diseases, such as pulmonary fibrosis.
- Useful for monitoring disease progression or response to treatment.
DLCO testing for COPD
Learn more
What is the difference between spirometry and a pulmonary function test (PFT)?
A spirometry test is a specific type of pulmonary function test. Spirometry measures the lungs volume (or how much) and flow (how quickly) the patient can move air into and out of their lungs. Spirometry will tell the tester if the patient has an obstruction, restriction, a mixed defect, or has normal lung flow.
Spirometry does NOT look at gas exchange and does not provide absolute lung volumes (RV, FRC, and TLC).
Diffusion capacity or transfer factor of the lung for carbon monoxide (CO) is known as a DLCO test. This measures the gas exchange and is used in conjunction with spirometry to provide a differential diagnosis. DLCO is also used to assess disease severity and is one of the best correlates of emphysema in COPD.
The final component that completes the full PFT exam is measuring absolute lung volume (RV, FRC, and TLC). This is completed by measuring body plethysmography, gas dilution, or nitrogen washout. Lung volumes are commonly used for the diagnosis of restriction. In obstructive lung disease, they are used to assess for hyperinflation. The changes in lung volumes can also be seen in a number of other clinical conditions.
Want to learn more about adding PFT and spirometry to your practice?
Get a live demo today!
What will lung volumes show you?
Lung volumes are used to diagnose restrictive lung disease and to assess for hyperinflation in obstructive lung disease. With restrictive lung disease, TLC, VC, and RV may be decreased due to the inability of the lungs to expand properly.
The lungs are restricted from fully expanding and filling with air. In an obstructive disease such as COPD, patients may develop hyperinflation, which will result in a higher than normal TLC and RV. Conversely, IC will be decreased due to air trapping in the lungs.
Lung volume testing with the EasyOne Pro LAB provides fast, accurate, and fully automated measurements without the need for a body box. Its compact design and TrueFlow and TrueCheck technologies ensure reliable results for comprehensive lung assessment in most clinical settings.
EasyOne Pro LAB - Portable DLCO, MBW, Lung Volumes, LCI and Spirometry
Discover the benefits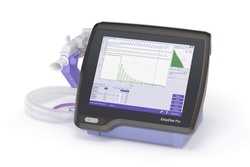
When would you use DLCO testing?
Diffusion capacity testing (DLCO) is best used to pinpoint the type of respiratory disease. It is completed after spirometry when an obstruction or lung volume issues are predetermined.
- Differentiating emphysema from obstructive bronchitis and chronic asthma
- Assessment of COPD
- Detection of pulmonary vascular disease
- Assessment of shortness of breath (SOB)
- Disability/Impairment evaluations for ILD or COPD
Specifications
Standards & Recommendations
Quality, electrical, medical devicesIEC 60601-1, IEC 60601-1-2, IEC 62304, IEC 62366, ISO 13485, ISO 14971, ISO 26782, ISO 23747FDA510(k) market clearanceMDD 93/42/EECCE markedAssociations & InstitutesATS/ERS 2022, 2019 & 2005 Spirometry Standards, NIOSH, OSHA, SSA Disability ATS/ERS 2017 & 2005 DLCO StandardsLanguages
Graphical user interfaceChinese, Croatian, Danish, Dutch, English, Finnish, French, French (Canada), German, Italian, Japanese, Norwegian, Polish, Portuguese, Portuguese (Brazil), Russian, Spanish, Swedish, Turkish, VietnameseGas specification
DLCOThe DLCO test requires a gas mixture within an accuracy range of <2%.
- 10% helium, accuracy ±10%
- 0.3% carbon monoxide, accuracy ±10%
- 18% to 25% oxygen
- balance nitrogen
MBW100% oxygenTechnical
Printing optionsPCL standard, direct to printer or via networkData managementEasyOne Connect (SQLite, MS SQL Server)ExportHL7, XML, GDT, via USB, LAN networkData linksEthernet port, USB, option to upgrade to WLANNo. of tests>10,000 testsAge rangeSpirometry ≥4 years, DLCO ≥6 years, MBW ≥4 years or >18kg
Dimensions27 x 33.5 x 27 cm (H x W x D), 8 kg
10.6 x 13.2 x 10.6”, 17.65 lbDisplayTouch display size: 12.1”
Resolution: 1024 x 768 pixelsDevice classificationProtection class I; Type BF applied partOperating conditionsTemp 5-40°C/41-104°F
Rel. humidity 15-90%,
no condensation
Atmosph. pressure 620-1060 hPaPower ConsumptionUp to 80 VAParameters
FVCATI, BEV, EOTV, FEF10, FEF25, FEF25-75, FEF25-75_6, FEF40, FEF50, FEF50/FVC, FEF50/VCmax, FEF60, FEF75, FEF75-85, FEF80, FET, FET25-75, FEV.25, FEV.5, FEV.5/FVC, FEV.75, FEV.75/FEV6, FEV.75/FVC, FEV.75/VCmax, FEV1, FEV1/FEV6, FEV1/FVC, FEV1/FVC6, FEV1/VC, FEV1/VCmax, FEV1Q, FEV3/FVC, FEV3/VCmax, FEV3, FEV6, FVC, MEF20, MEF25, MEF40, MEF50, MEF60, MEF75, MEF90, MMEF, MTC1, MTC2, MTC3, MTCR, PEF, PEFT, t0, VC, VCmaxFVLATI, BEV, CVI, E50/I50, EOTV, FEF10, FEF25, FEF25-75, FEF25-75_6, FEF40, FEF50, FEF50/FVC, FEF50/VCmax, FEF60, FEF75, FEF75-85, FEF80, FET, FET25-75, FEV.25, FEV.5, FEV.5/FVC, FEV.75, FEV.75/FEV6, FEV.75/FVC, FEV.75/VCmax, FEV1, FEV1/FEV6, FEV1/FIV1, FEV1/FIVC, FEV1/FVC, FEV1/VC, FEV1/VCmax, FEV3/FVC, FEV3/VCmax, FEV1Q, FEV3, FEV6, FIF25, FIF 25-75, FIF50, FIF50/FEF50, FIF75, FIV.25, FIV.5, FIV1, FIVC, FVC, MEF20, MEF25, MEF40, MEF50, MEF60, MEF75, MEF90, MIF25, MIF50, MIF75, MMEF, MMIF, MTC1, MTC2, MTC3, MTCR, PEF, PEFT, PIF, t0, VC, VCmaxSVCERV, IC, IRV, Rf, VC, VCex, VCin, VCmax, VTMVVMVV, MVV6, MVVtime, Rf, VCext, VTDLCOBHT, COHb, ColBarVol, CO Conc, HE Conc, O2 Conc, Anatomic Dead Space, System Dead Space, Discard Volume, DLadj, DLadj/VA, DLCO, DLCO/VA (KCO), ERV, FA CO, FA HE, FE CO, FEV1/FVC, FI CO, FI HE, FRC sb, FRC Cor, Hb, tI, Kroghs K, PaO2, RV sb, RV Cor, RV/TLC sb, RV/TLC Cor, TLC sb, TLC Cor, TLCO, VA sb, VA Cor, VCext, VCmax, Vd, VI, VTMBWCEV, CEV5, Anatomic Dead Space, System Dead Space, ERV, FRCbase, FRCextrapol, FRCmb, IC, IRV, LCI, LCI5, LCIao, MO, MR1, MR2, Rf, RVmb, RV/TLCmb, TLCmb, VAmb, VC, VCex, VCin, Vd, VT, VT/FRCmb, VT/kgPredicted Normal Values – Spirometry
GLIStanojevic 2009, Quanjer 2012, Bowerman 2023 (Global GLI)North AmericaNHANES III (Hankinson) 1999, Knudson 1983, Knudson 1976, Crapo 1981, Morris 1971 & 1976, Hsu 1979, Dockery (Harvard) 1993, Polgar 1971, Gutierrez (Canada) 2004, Eigen 2001, Cherniak 1972Latin AmericaChile 2010, Chile (Pediatrics) 1997, Jones 2022, Pereira 1992, Pereira 2006/2008, Pereira-Prata 2018, Pérez-Padilla (PLATINO) 2006, Pérez-Padilla (Mexico) 2001, Pérez-Padilla (Mexico, Pediatrics) 2003EuropeERS (ECCS, EGKS, Quanjer) 1993, Garcia-Rio (SEPAR) 2013, Falaschetti 2004, Forche (Austria) 1988 & 1994, Klement (Russia) 1986, Roca (Spain, SEPAR) 1982, Rosenthal 1993, Sapaldia (Switzerland) 1996, Vilozni 2005, Zapletal 1977, Zapletal 2003Europe ScandinaviaHedenström (Sweden) 1985/1986, Gulsvik (Norway) 1985, Berglund Birath (Sweden) 1963, Langhammer (Norway) 2001, Finnish 1982/1998, Nystad 2002, Koillinen 1998, 2001, Kainu (Finland) 2016AustraliaHibbert 1989, Gore Crockett 1995AsiaChhabra (India) 2014, Dejsomritrutai (Thailand) 2000, (Indonesia) 1992, IP (China, HongKong) 2000 & 2006, JRS 2001 & 2014AfricaMengesha (Ethiopia) 1985Predicted Normal Values – DLCO
North AmericaAyers 1975, Burrows 1961, Crapo 1981 & 1982, Knudson 1987, McGrath & Thompson 1959, Miller 1980, Gutierrez (Canada) 2004, NHANES (Neas) 1996, Polgar 1971Latin AmericaVazquez Garcia (ALAT) 2016, Gochicoa 2019EuropeStanojevic (GLI) 2017, ERS ECCS/EGKS 1993, Zapletal 1977, Roca 1990 & 1998, Hedenström 1985 & 1986, Gulsvik 1992, Klement (Russia) 1986OtherPereira 2008, Thompson 2008, Kim 2012, Chhabra (India) 2015), Ip (China, HongKong) 2007, JRS (Japan) 2001Predicted Normal Values – MBW
EuropeVerbanck 2012Flow/Volume Sensor
Measurement PrincipleUltrasonic transit timeFlow Range±16 l/sFlow Resolution1 ml/sFlow Accuracy (except PEF)±2.5% or 0.02 l/PEF Accuracy±5% or 0.200 l/sVolume Accuracy±2.5% or 0.050 lMVV Accuracy±5% or 5 l/minResistance<1.5 cm H2O/l/s at 14 l/sGas-Sensor
COCO2TypeNon-dispersive infraredRange0 to 0.35%0 to 10%Resolution0.0001% (1 ppm)0.005%Accuracy± 0.0015% (15 ppm)± 0.0015% (15 ppm)Tracer Gas Sensor
HeliumCO2TypeUltrasonic transit timeRange0 to 50%0 to 100%Resolution0.02%0.1%Accuracy0.05%0.2%Ordering Information Devices
Part NumbersDescription3100-1EasyOne Pro LAB Respiratory Analysis SystemOrdering Information Accessories
Part NumbersDescription2050-1Case of 50 Spirette mouthpieces2050-5Case of 200 Spirette mouthpieces2050-10Case of 500 Spirette mouthpieces3050-1Case of 50 DLCO Barriette for EasyOne Pro and LAB3050-2Case of 100 DLCO Barriette for EasyOne Pro and LAB3150-1Case of 40 FRC Barriette for EasyOne Pro LAB3150-2Case of 80 FRC Barriette for EasyOne Pro LAB
Spirette FA Pro/LAB Specifications
Product
ProductSpirette FA Pro/LABCompatible with ndd productsEasyOne Pro and EasyOne Pro/LAB with Software Version V03.07.01.09 (or higher)Compatible with filterU.S. & International customers: To learn more about compatible inline filter solutions available in your region, please use the contact us form.Transport and storage conditionsTemperature: –20 to 50° C Humidity: 5 to 95% Pressure: 400 to 1060 hPaDimensions31 x 38 x 141 mmWeight11gMaterial Spirette FA Pro/LABLinear Low-Density Polyethylene, Polypropylene (LLDPE and PP)Material BagPolyethylene