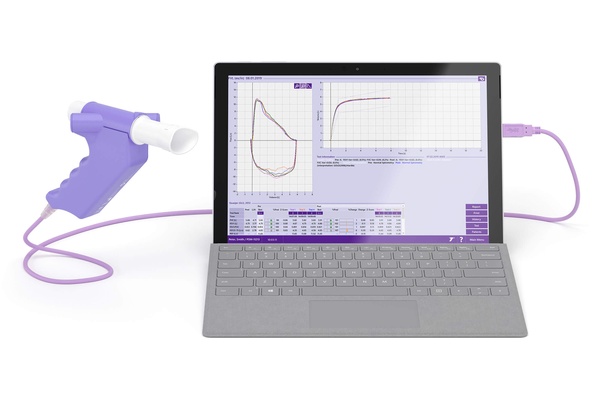
Calibration-free & Quick Spirometry
TrueFlow™ technology, high-quality & optimized components and Swiss precision-manufacturing deliver quick & accurate results without calibration.Quality Feedback & Interpretation
Simplify testing using the instant test quality feedback and interpretation platform.Real-Time Animated Graphs and Incentives
Interactive software displays, animated incentive screens and real-time graphs encourage child and adult patients to help them achieve maximum results.EMR Connectivity & Customized Settings
The flexibility of settings permits customized solutions including EMR/EHR integration to meet your individual needs.
Why our customers love us
- My clinical research on asthma and COPD happens at the point of care in some of the most remote, resource limited places in the world. Because of this, I need highly portable, very durable diagnostic devices reporting consistent and accurate results even under the toughest conditions. I use ndd’s devices because I know no matter the condition, the device will report accurate results.
- Our academic activities related to trainings and continuous learning for the specialists that take care of the respiratory health in our region, are supported by new technologies. Our experience with ndd devices has been very positive because they adapt to our necessities, they are always updated regarding new standardization and technological advances.
Research studies show reliable sensor performance under various conditions
Get optimal results with Easy on-PC, which is designed for maximum robustness under various conditions.
- Highly resistant to shock and vibration
- Strong cable protection
- Works in diverse environments
- Completely calibration-free

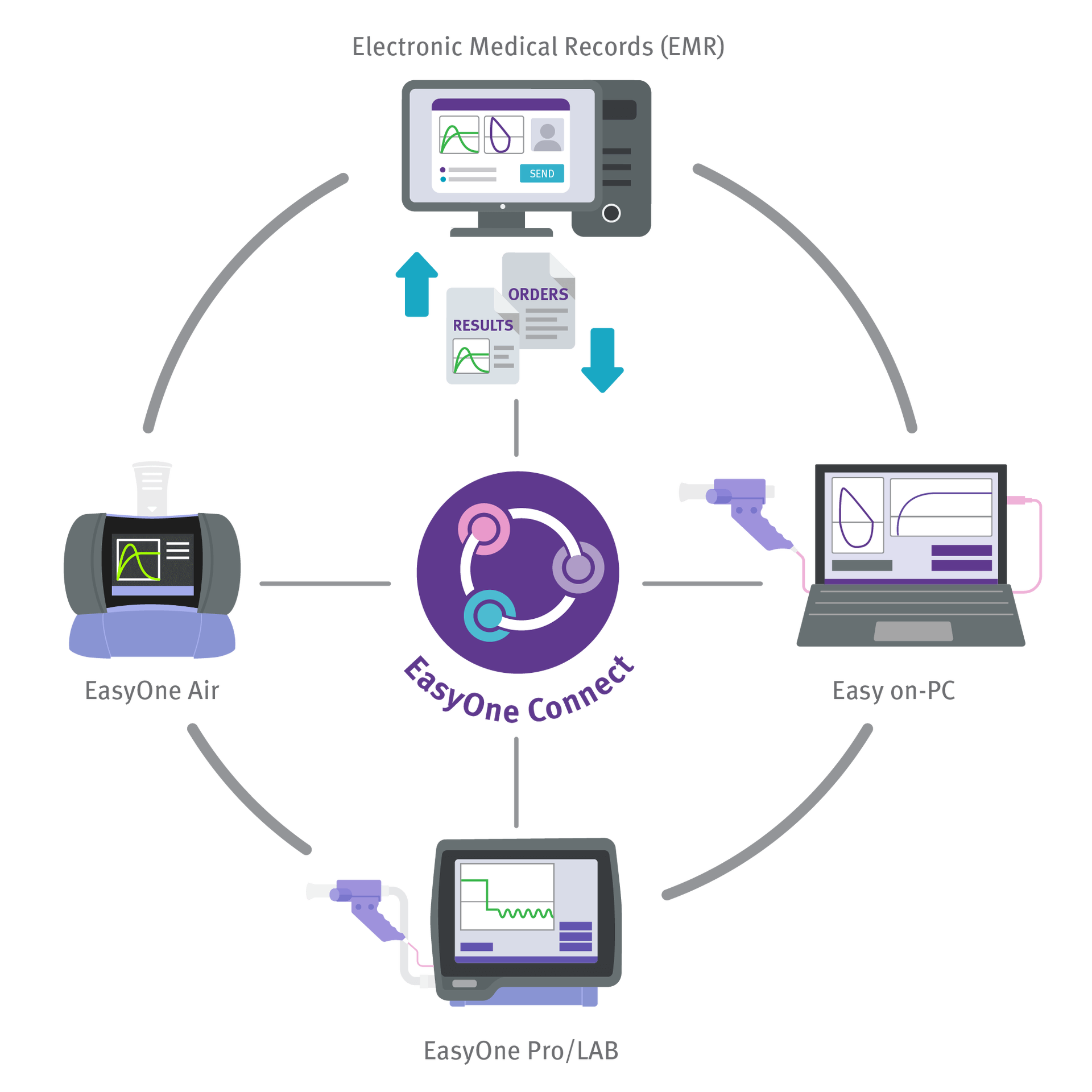
Powered by EasyOne Connect - ndd's Integrated Software Platform
The Easy on-PC spirometry sensor is directly connected via USB to your workstation where the EasyOne Connect software is installed. This software includes all advanced spirometry functionality at no extra charge and fulfills ATS/ERS 2005 and 2019 standards. Features include:
- QC-grading and result interpretation
- Powerful commenter functionality
- Encouraging pediatric incentives
- Customized reports
- Custom provocation protocols
- Large selection of predicted sets (%Pred, Z-score, and LLN)
- Import of external PFT results
For more information, please visit the EasyOne Connectproduct page.

Powerful EMR EHR Integration Engine
EasyOne Connect software includes powerful EMR EHR interoperability and IT management functionality for streamlined workflows. Features include:
- Proven EMR integrations
- Supports integration standards - HL7, XML, API, GDT, etc.
- Active directory integration
- SQL Server database
For more information, please visit the EMR EHR interoperability solutions page.
Worldwide Compliance with Standards
Stay compliant with ATS/ERS standards and specific country regulations.
- Fully conformant to ATS/ERS 2005 and 2019 and ISO standards
- Worldwide approvals
- CE Approval
- FDA Approval
- China Approval
Click here for a complete list of ndd certificates.

TrueFlow™ - Trusted Ultrasound Technology
Stop worrying about calibration or accuracy of flow measurements. TrueFlow™ is the only ultrasound technology proven to be accurate for a lifetime for flow and volume measurements.
- Proven long-term stability
- Contact and resistance-free measurement
- Excellent accuracy and robustness
- Patented technology
To review case studies and additional information about our proven technology, please click here.
Infection Control
The EasyOne Filter solutions provide additional protection for those who wish to include a filter when performing tests.
- The EasyOne Filter keeps the ambient environment clean for technicians and patients.
- The ndd breathing mouthpiece protects the flow sensor from contamination.
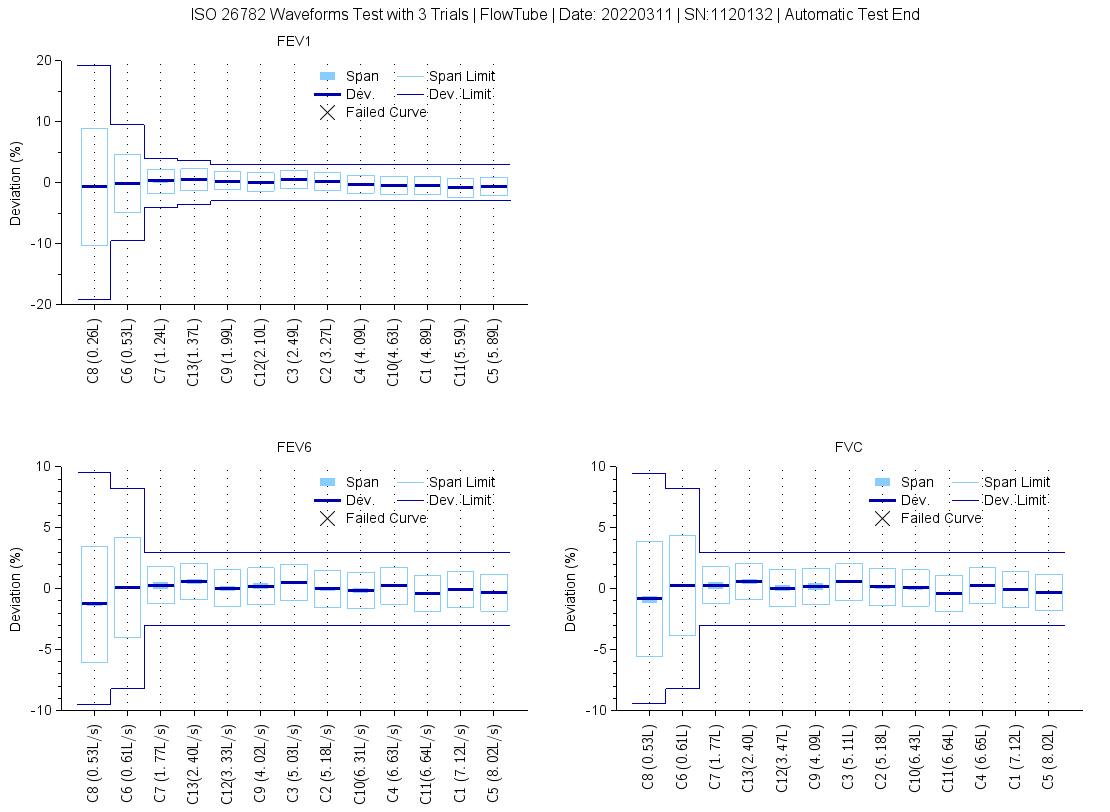
- Fully passes 13 Waveform test as required by the 2019 ATS/ERS standard.
- Available for most testing environments where filters are required.
Avoid cross-contamination and keep cleaning to an absolute minimum:
- All parts exposed to the patient’s breath are single-patient-use.
- The sensor is protected from contamination by the ndd breathing mouthpiece.
- Only simple surface cleaning is required for the ndd device.
- No special storage conditions are required for single-patient use Spirettesand FlowTubes (ndd breathing mouthpieces).
Visit our Infection Control page for more information.
Downloads
 Software
Software

- EasyOne Connect V03.09.07.05Release date: 2025-07-17Related documents
 Application Notes
Application Notes

 Brochures
Brochures

 Certificates
Certificates

 Manuals
Manuals

FAQs
Which EHR vendors can ndd integrate with?
ndd can integrate with any EHR vendor that can support a bi-directional HL7 orders/results lab interface.
What kind of integration options does ndd offer?
ndd offers a file-based integration utilizing an SFTP or network-based location for file communication with an EHR vendor. HL7 is the standard data structure that ndd utilizes but can support other data formats as well. The use of other interface engines can be utilized to complete an integration project.
What is the process for integrating your software with our existing systems?
The first step for an integration project is to complete the EMR integration request form here: https://nddmed.com/f/emr. An integration specialist will then reach out to you with the next steps.
How long does the integration process typically take?
Duration depends on the IT team of the facility. The ndd side of the integration process can be completed within a few hours for standard integrations. EasyOne Connect software is simply configured to point to the SFTP or network-based location where files are being placed.
How does ndd ensure that data is securely transferred during integration?
EasyOne Connect software utilizes AES 256 encryption. SFTP has its own security protocols built into it. If a network-based location is utilized, it will follow the protocols of the facility.
Can patient data and test results be accessed across multiple laptops and desktops within a clinic?
Yes, EasyOne Connect offers an optional centralized patient database feature, enabling seamless access to patient orders and results across all instances of the software, irrespective of physical location. Customers can leverage their on-premise SQL servers or MS Azure environments to ensure data accessibility and consistency.
Does ndd offer user management within the software?
EasyOne Connect provides flexible options for managing users and their access. User accounts can be created and managed directly within EasyOne Connect for simple permission control. For more advanced management, the software supports integration with Active Directory for using your existing directory service accounts, groups, and permissions. EasyOne Connect also supports optional single sign-on (SSO) capability. If you would like to review the Active Directory Application Note, please contact [email protected].
Can ndd provide examples of successful integration with other clients?
ndd has integrated with all major vendors and can provide examples of successful integrations with various healthcare providers and organizations. You can contact our integration department via email at [email protected] for more information.
How does ndd handle updates and maintenance to the integration?
ndd does not handle the maintenance and upgrades as the software is installed locally on the client’s machine. We do, however, offer a silent installer package that our support team can assist you with. This silent installer can be executed via a script through active directory commands depending on your facility. It is the responsibility of the facility to implement any future updates. There is no licensing fee associated with the software and/or the updates.
Are there any additional costs associated with integration?
ndd does not charge for standard integration. In the event custom development is needed, ndd will provide a statement of work before a project begins. Almost all EHR vendors will charge a fee that the client is responsible for.
What are the HL7 specifications?
HL7 specifications can be found here.
What are the database options?
- Local SQLite file-based database.
- On-premise Microsoft SQL Server database.
- Azure cloud-hosted Microsoft SQL Server database.
Please see the application note for additional information.
Who is responsible for setting up and hosting the SFTP and/or network-based location?
The facility is responsible for setting up and hosting either option. ndd requires a username and password if using an SFTP environment.
What results are received in the HL7 message?
The result HL7 message will consist of a combination of discrete results and an embedded base64 PDF attachment for the PFT report. If you prefer, we also offer the option of including a reference link instead of the embedded PDF.
Is a server required?
No, a server is not required.
What kind of support does ndd offer during and after the integration process?
You can schedule a meeting with an integration engineer from 8 a.m. to 5 p.m. ET Monday through Friday. ndd can accommodate meetings outside of that timeframe if planned accordingly. The integration team can be reached via email at [email protected]. The support team can also be reached via email at [email protected] or by phone at (978) 470-0923.
Which predicted or normal values are supported?
You can find the product specification on the respective product pages. The specification contains a list of all supported normal values.
To get more detailed information about the supported parameters, age range, ethnicities and height range of each supported predicted set, please click here.
Where can I download the software for Easy on-PC?
The Easy on-PC software, EasyOne Connect, can be downloaded free of charge directly from our website. Click here for the download.
Can the software be downloaded onto multiple devices?
Yes, the EasyOne Connect software can be downloaded onto as many laptop, PC or tablet devices as desired. There are no license requirements.
Is a laptop, PC or tablet provided with the system?
No, a laptop, PC or tablet must be purchased separately. The device specifications can be found in the Requirements PC/Laptop section under Specifications on the Easy on-PC page.
What are the minimum computer requirements?
Please consult Section 1.11 in the manual here for the latest computer requirements. Note that for the Easy on-PC latest version, V02.02.00.14 or greater, the following operating systems are no longer supported: Windows XP SP3 and Windows Vista.
What are the tablet requirements?
The general requirements for notebooks and tablets the following:
- USB interface
- Windows operating system (32 or 64 bit)
- Hard disk capacity of 1 GB installation and up to 4 GB of data
- RAM 2 GB
The embedded versions of Windows are no longer compatible.
What incentive screens are available in the EasyOne Connect software?
The EasyOne Connect software for the Easy on-PC offers a variety of animated screens for children as well as adults. These incentive screens, in addition to the real-time curves, encourage the patients to help them achieve high quality spirometry results. Here are some examples of the pediatric incentive screens available:


How do I backup my patient data?
Data can safely be exported by using the “Export Data” feature or “Backup” feature within the Storage tab in the configuration.
Where can I purchase the Easy on-PC?
All our products can be purchased through our authorized distribution partners. For US customers, please contact us to receive more information on finding your local dealer. International customers please click here for a list of dealers in your country.
Do I need to calibrate the Easy on-PC spirometer?
No. All EasyOne products are designed to not require calibration. ndd’s patented TrueFlow technology is the only technology proven to remain stable over its lifetime. A number of independent papers have been published that confirm this long-term stability.
For organizations like NIOSH/OSHA or Social Security/Disability, where calibration checks are required, ndd offers a 3-Liter calibration syringe. A calibration check (cal-check) is not to be confused with calibration. A cal-check simply validates that the device is within calibration limits. Unlike calibration, a cal-check does not adjust the calibration values.
Device not connected. Easy on-PC not recognized in EasyOne Connect
If your Easy on-PC is not recognized in the EasyOne Connect Software, ensure the device is connected directly to the PC without a USB hub.
If the error continues, the cause may be the USB cable.
Cable damage can lead to intermittent or consistent connection issues.
Cable damage can be caused by improper storage or handling.
Do not wrap the cable around the handle of the device.
Keep the cable away from sharp edges.
If you notice any damage to the cable, discontinue use and contact ndd service and support.
The cable can be replaced at ndd or at a certified repair location.
Learn more by watching the video below.
How long does a spirometry test take?
A spirometry test typically takes 10 to 15 minutes, depending on the patient’s ability to follow instructions and complete the required breathing maneuvers. The test involves taking a deep breath and forcefully exhaling into a spirometry device multiple times to achieve accurate results.
Healthcare providers may repeat the test after administering a bronchodilator to assess lung function improvement. Proper coaching and technique are essential for reliable measurements.
Learn how to perform a spirometry test step by step.
Read the blog.
How to backup my database in EasyOne Connect
In EasyOne Connect, go to: Utilities > Export Data > tick Patient Data > click Export. Save the file in the preferred location.
Learn more by watching the video below.
How to coach a patient to perform spirometry
The typical spirometry exam is a three-phased process – each with their own coaching techniques to help patients produce an effective test.
- Phase 1
In the first phase, the goal is to instruct the patient on the proper techniques to apply their mouth to the spirometry mouthpiece. Best results are achieved when the patient takes a deep, deep breath, placing the mouthpiece between their teeth, and sealing the mouthpiece with their lips. - Phase 2
The second phase is the BLAST. This is when the patient will blow out as hard and as fast as possible. Coaching a patient in this phase is best achieved by developing a rapport with them prior to the exam, as the tester will learn which level of coaching is best for that individual patient. - Phase 3
The final phase is the remaining 5 to 6 seconds of exhalation. In this phase, you’ll quietly encourage the patient to “keep blowing” to complete the exhalation.
- Phase 1
How to create a new patient database in EasyOne Connect
A new database can be created in Utilities > Configuration > General tab > Storage tab, click New, Select File Based, click New Name the file, click Save.
Setting a password is optional. This password is needed to open the database and cannot be recovered. Make sure to remember the password, otherwise the data cannot be accessed.
Click OK, Click save.
The software will refresh and the new database will be loaded.
Learn more by watching the video below.
How to export a single patient database
ndd Service and Support may request a single patient database for review if there are any questions about test performance.
A single patient database can be exported from EasyOne Connect in the following:
Patients > Select the patient > Click the double arrows to expand the menu > Select Database > Click Save File as and save the file > Click Export.
How to export the XML database from EasyOne Connect and convert it to a CSV file
The EasyOne Connect Patient Data can be exported as an XML file and converted to a CSV file. First locate the EasyOne Connect Data Export application note found on our website.
- Click on the link in the app note to download the zip file, which contains a library of required files needed for the conversion.
- Extract the files from the ZIP folder.
- In Easy One Connect export the XML file of the database. Go to: Utilities > export XML.
- Save the XML file in the XML input folder in the conversion file library.
This will export the entire database. Single tests can also be exported as an XML file in the patient history.
The Conversion File library contains several conversion options that include different parameters.
- Double click on a bat file to run the conversion. This will convert any files in the XML input folder.
Press any key to close out of the conversion window.
The converted CSV file is found in the CSV output folder.
Learn more by watching the video below.
How to import a patient database
A database can be imported into the currently selected database in Utilities Configuration, the General tab, the Storage tab, click Import, Select File Based.
Click select. Select the database file to be opened.
Click Open. Click OK.
When the import is complete, click Save.
The chosen database will be merged with the currently selected database.
Learn more by watching the video below.
How to locate the currently selected database in EasyOne Connect
In EasyOne Connect, go to: Utilities > Configuration > General (tab) > Storage (tab) and there it is listed under database name. Default location on the PC:
C:\ProgramData\ndd\EasyOne Connect\EasyOneConnect.sqlite.
Learn more by watching the video below.
How to select a different patient database in EasyOne Connect
A different database can be selected in Utilities > Configuration > General tab > Storage tab.
Click Select > Select File Based.
Click Select. Select the database file to be opened. Click Open Review the database path. Click OK Click Save.
The software will refresh.
Learn more by watching the video below.
How to troubleshoot a "baseline setting failed" on the Easy on-PC
Each time a test is started, the baseline is set. The software will prompt you to block the Spirette for this step so there is no airflow. Passing the baseline setting is required before the test can start. If the baseline fails, there may be an error stating:
“baseline setting failed” “Check Spirette insertion” “Device initialization failed”
Here are a few steps to try when the baseline setting error occurs.
- First, ensure the Spirette is blocked and fully inserted.
- Open the Spirette wrapper halfway from the bottom, leaving the top part wrapped.
- Insert the Spirette fully align the arrows.
- Push the Spirette in until there are no gaps.
- Avoid any airflow, including vents, fans, or windows.
- The end of the Spirette can also be blocked by a hand.
- If the error still occurs, try changing the Spirette out with a new one.
If the error still occurs after changing the Spirette, try to reset the amplitude.
Resetting the amplitude on the device may resolve the baseline setting errors.
To reset the amplitude, follow these steps:
- Start a test and attempt to set the baseline.
- When the baseline setting fail error appears, remove the Spirette and reinsert it at a 90° rotation. Intentionally incorrect.
- Click Retry. There will be another error.
- Insert the Spirette correctly again and restart the test to check if that resolved the error.
These steps may need to be attempted a few times.
Learn more by watching the video below.
What are examples of obstructive lung diseases?
A few examples of obstructive lung disease include:
- Chronic Obstructive Pulmonary Disease (COPD)
- Emphysema
- Chronic Bronchitis
- Asthma
- Bronchiectasis
What are examples of restrictive lung disease?
A restrictive lung disease is exactly that – one where a restriction occurs in the respiratory system.
A few examples of restrictive lung disease include:
- Interstitial lung diseases such as idiopathic pulmonary fibrosis
- Sarcoidosis
- Obesity
- Scoliosis
- Neuromuscular diseases such as muscular dystrophy or ALS
What are the important parameters in spirometry?
A spirometry test will produce a series of results or parameters that help medical professionals easily complete this exam. The three most important spirometry parameters are the FEV1, FVC, and FEV1/FVC ratio parameters.
A simple guide to reading spirometry results.
Get the tips from Buddy
What indictations are used for spirometry?
There are multiple patient-symptoms or medical conditions that justify the use of spirometry. A few of the most common indicators include:
- To manage asthma and COPD patients
- Evaluate shortness of breath
- Perform surveillance for occupational-related lung disease
- Evaluate former or current smokers over the age of 45 for COPD
- Classification of COPD. (COPD is an umbrella term that covers multiple respiratory diseases including chronic bronchitis, emphysema, and idiopathic pulmonary fibrosis (IPF))
- Monitor disease progression
- Measure response and progress due to treatment, medication, and respiratory therapy
- Smoking cessation
Tips from leading experts on the value of spirometry.
Watch the webinar
What is a diagnostic spirometer?
A diagnostic spirometer is a medical device that is used to measure lung function. It can be a portable or stationary spirometry device that is operated by a medical professional who is specially trained in proper testing procedures and techniques to produce a more consistent and reliable result.
ndd EasyOne spirometry solutions
Speak to a clinical specialist today!
###
What is FEV1 in spirometry and what does it tell you?
FEV stands for Forced Expiratory Volume. The FEV1 parameter equates to the volume that is exhaled after one second. It is intended to measure the severity of an obstruction – with average FEV1 results measuring two to four liters. A lower FEV1 result equates to higher obstruction.
A simple guide to reading spirometry test results.
Get the tips from Buddy
What is FVC in spirometry and what does it tell you?
FVC stands for Forced Vital Capacity. FVC determines the total exhaled volume of air. FVC tells the spirometry tester how much air volume the patient can exhale. The FVC will fall when the patient can’t inhale deeply or can’t exhale completely. The average FVC result for an elderly man is about four liters (one gallon) of air. The intent of this parameter is to detect restriction.
A simple guide to reading spirometry test results.
Get the tips from Buddy.
What is spirometry used for?
Spirometry is used to diagnose and monitor lung diseases, assess pulmonary function, and support occupational health screenings. Spirometry helps healthcare providers to:
- Diagnose respiratory conditions like COPD and asthma
- Monitor disease progression and treatment effectiveness in patients with chronic lung conditions
- Evaluate lung function in workplace health programs, particularly for individuals exposed to dust, chemicals, or other respiratory hazards
By using a diagnostic spirometer, medical professionals can detect lung diseases early, track changes over time, and ensure workplace safety.
Ready to add spirometry testing to your practice?
Discover more now.
What is spirometry?
Spirometry is the primary method of assessing overall lung function. It works by measuring the volume of air that a patient can forcefully expel from the lungs after inhaling as much as possible. This non-invasive test is completed by medical professionals and is essential for diagnosing and monitoring respiratory conditions like COPD, asthma, and other lung diseases.
Using a diagnostic spirometer, a trained healthcare professional can assess lung health, detect early signs of disease, and guide treatment decisions.
Discover our advanced spirometry solutions here.
Book a demo today!
What is the difference between spirometry and a pulmonary function test (PFT)?
A spirometry test is a specific type of pulmonary function test. Spirometry measures the lungs volume (or how much) and flow (how quickly) the patient can move air into and out of their lungs. Spirometry will tell the tester if the patient has an obstruction, restriction, a mixed defect, or has normal lung flow.
Spirometry does NOT look at gas exchange and does not provide absolute lung volumes (RV, FRC, and TLC).
Diffusion capacity or transfer factor of the lung for carbon monoxide (CO) is known as a DLCO test. This measures the gas exchange and is used in conjunction with spirometry to provide a differential diagnosis. DLCO is also used to assess disease severity and is one of the best correlates of emphysema in COPD.
The final component that completes the full PFT exam is measuring absolute lung volume (RV, FRC, and TLC). This is completed by measuring body plethysmography, gas dilution, or nitrogen washout. Lung volumes are commonly used for the diagnosis of restriction. In obstructive lung disease, they are used to assess for hyperinflation. The changes in lung volumes can also be seen in a number of other clinical conditions.
Want to learn more about adding PFT and spirometry to your practice?
Get a live demo today!
What is the FEV1/FVC Ratio in spirometry and what does it tell you?
The FEV1/FVC ratio is typically expressed as a percentage (such as 75%). Nearly three-fourths of lung volume can normally be exhaled during the first second of the spirometry test. As such, the normal ratio falls between 65 to 85 percent. The range of normal FEV1/FVC ratio is determined by the patient’s age.
A simple guide to reading spirometry test results.
Get the tips from Buddy.
Specifications
Standards & Recommendations
Quality, Medical Devices & ElectricalIEC 60601-1, IEC 60601-1-2, IEC 62304, IEC 62366, ISO 13485, ISO 14971, ISO 26782, ISO 23747FDA510(k) market clearanceMDR (EU) 2017/745CE-markedStandards & institutesATS/ERS 2005 spirometry standard,
ATS/ERS 2019 spirometry standard,
ATS/ERS 2022 interpretation strategies, NIOSH, OSHA, SSA DisabilityLanguages
User InterfaceChinese, Croatian, Danish, Dutch, English, Finnish, French, French (Canada), German, Italian, Japanese, Norwegian, Portuguese, Portuguese (Brazil), Russian, Spanish, Swedish, Turkish, VietnameseTechnical
Printing OptionsDirect to printer or via networkData managementEasyOne Connect (SQLite, MS SQL Server)Export/EMRHL7, XML, GDTNo. of tests>10,000 testsAge rangeSpirometry ≥4 yearsDevice classificationType BF applied partOperating conditionsTemp 0-40 °C / 32-104 °F
Rel. humidity 5-90 %
Atmosph. pressure 620-1060 hPARequirements for PC
Hard disk capacityInstallation/system 1 GB
Data up to 4 GBRAM2 GBOperating systemMicrosoft Windows 7, Microsoft
Windows 8 and 8.1 (32 bit and 64 bit), Microsoft Windows 10 (32 bit and 64 bit), Microsoft Windows 11Parameters
FVCATI, BEV, EOTV, FEF10, FEF25, FEF25-75, FEF25-75_6, FEF40, FEF50, FEF50/FVC, FEF50/VCmax, FEF60, FEF75, FEF75-85, FEF80, FET, FET25-75, FEV.25, FEV.5, FEV.5/FVC, FEV.75, FEV.75/FEV6, FEV.75/FVC, FEV.75/VCmax, FEV1, FEV1/FEV6, FEV1/FVC, FEV1/FVC6, FEV1/VC, FEV1/VCmax, FEV1Q, FEV3/FVC, FEV3/VCmax, FEV3, FEV6, FVC, MEF20, MEF25, MEF40, MEF50, MEF60, MEF75, MEF90, MMEF, MTC1, MTC2, MTC3, MTCR, PEF, PEFT, t0, VC, VCmaxFVLATI, BEV, CVI, E50/I50, EOTV, FEF10, FEF25, FEF25-75, FEF25-75_6, FEF40, FEF50, FEF50/FVC, FEF50/VCmax, FEF60, FEF75, FEF75-85, FEF80, FET, FET25-75, FEV.25, FEV.5, FEV.5/FVC, FEV.75, FEV.75/FEV6, FEV.75/FVC, FEV.75/VCmax, FEV1, FEV1/FEV6, FEV1/FIV1,FEV1/FIVC, FEV1/FVC,FEV1/VC, FEV1/VCmax, FEV3/FVC,FEV3/VCmax, FEV1Q, FEV3, FEV6, FIF25, FIF 25-75, FIF50, FIF50/FEF50, FIF75, FIV.25, FIV.5, FIV1, FIVC, FVC, MEF20, MEF25, MEF40, MEF50, MEF60, MEF75, MEF90, MIF25, MIF50, MIF75, MMEF, MMIF, MTC1, MTC2, MTC3, MTCR, PEF, PEFT, PIF, t0, VC, VCmaxSVCERV, IC, IRV, Rf, VC, VCex, VCin, VCmax, VTMVVMVV, MVV6, MVVtime, Rf, VCext, VTPredicted Normal Values – Spirometry
GLIStanojevic 2009, Quanjer 2012, Bowerman 2023 (Global GLI)North AmericaNHANES III (Hankinson) 1999, Knudson 1983, Knudson 1976, Crapo 1981, Morris 1971 & 1976, Hsu 1979, Dockery (Harvard) 1993, Polgar 1971, Gutierrez (Canada) 2004, Eigen 2001, Cherniak 1972Latin AmericaChile 2010, Chile (Pediatrics) 1997, Pereira 1992, Pereira 2006/2008, Pérez-Padilla (PLATINO) 2006, Pérez-Padilla (Mexico) 2001, Pérez-Padilla (Mexico, Pediatrics) 2003EuropeERS (ECCS, EGKS, Quanjer) 1993, Garcia-Rio (SEPAR) 2013, Falaschetti 2004, Forche (Austria) 1988 & 1994, Klement (Russia) 1986, Roca (Spain, SEPAR) 1982, Rosenthal 1993, Sapaldia (Switzerland) 1996, Vilozni 2005, Zapletal 1977, Zapletal 2003Europe ScandinaviaHedenström (Sweden) 1985/1986, Gulsvik (Norway) 1985, Berglund Birath (Sweden) 1963, Langhammer (Norway) 2001, Finnish 1982/1998, Nystad 2002, Koillinen 1998, 2001, Kainu (Finland) 2016AustraliaHibbert 1989, Gore Crockett 1995AsiaChhabra (India) 2014, Dejsomritrutai (Thailand) 2000, (Indonesia) 1992, IP (China, HongKong) 2000 & 2006, JRS 2001 & 2014AfricaMengesha (Ethiopia) 1985Flow/Volume Sensor
TypeMeasurement principleMeasuring range±16 l/sFlow resolution4 ml/sFlow accuracy (except PEF)±2.5% or 0.020 l/sPEF accuracy±5% or 0.200 l/sVolume accuracy±2.5% or 0.050 lMVV accuracy±5% or 5 l/minResistance~ 0.3 cm H2O/l/s at 16 l/sSample Rate400 HzOrdering Information Devices
Part numberDescription2700-3Easy on-PC Spirometry SystemOrdering Information Accessories
Part numberDescription2050-1Case of 50 Spirette mouthpieces2050-5Case of 200 Spirette mouthpieces2050-10Case of 500 Spirette mouthpieces
Not available in all countries
Accessories

EasyOne Filter
It’s not just a filter. It’s the EasyOne Filter.