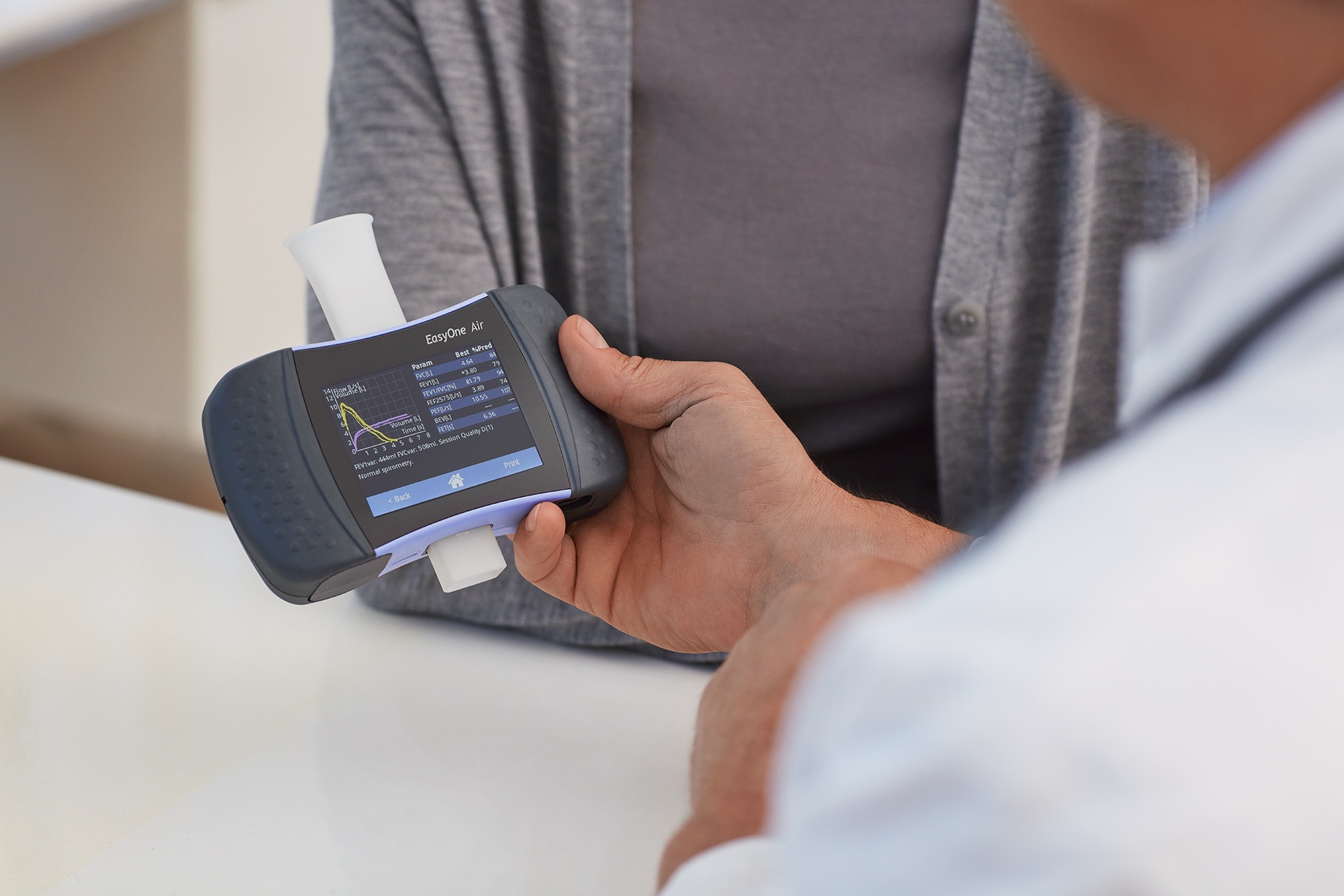
Flexible Use, Consistent Results
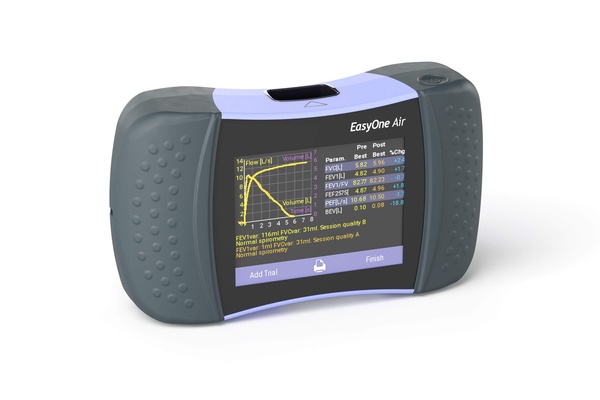
EasyOne Air can be used as a portable or as a PC spirometer. It delivers consistent results in various conditions and settings.Calibration-free TrueFlow™ Technology
Utilizing high-quality components and Swiss precision-engineering, TrueFlow™ delivers robust and reliable testing results without needing calibration.Color Touch Screen
View real-time graphs, quickly enter patient data and easily navigate with the high resolution color touch screen in the EasyOne Air.Bluetooth and EMR Connectivity
Easily connect to PC for real-time patient incentives, data exchange and EMR connectivity.
Ready to Take on Any Challenge
EasyOne Air can be trusted in various locations and environments to perform reliably.
- Reliable design tried and tested at large screening events
- Extremely robust TrueFlow™ measurement technology
- Performs up to 100 complete tests with one battery charge
- Proven completely calibration-free device
Choose Between Portable Mode and PC Mode
Use EasyOne Air in portable mode, where the data is stored and the graph is displayed directly on the device, or use it in PC mode for more options.
- Portable or PC mode
- Stable Bluetooth connection
- Real-time curve display
- Real-time data transfer
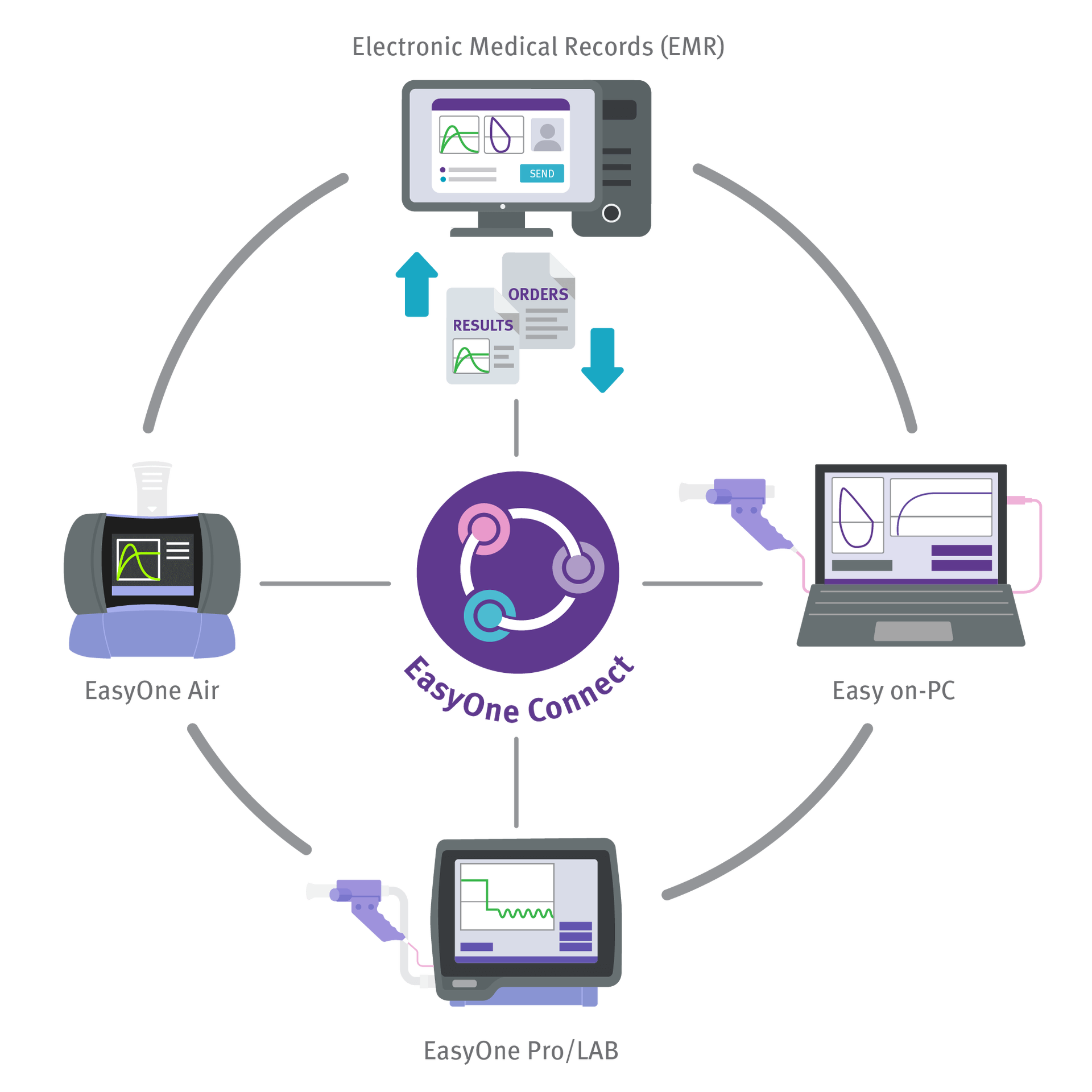
Connectivity to Printers, PCs and EMR
Easily integrate EasyOne Air into your existing clinical workflow. The device is equipped with Bluetooth and USB modules making connections to printers, a central database and EMR systems extremely easy and stable.
- Directly connect printers via USB or print via EasyOne Connect
- Synchronize data via USB or Bluetooth
- Integrate into all major EMR/EHR systems via HL7, XML, GDT, or API
- Reference projects to all major EMR systems available on request

Powered by EasyOne Connect - ndd's Integrated Software Platform
Start using EasyOne Air in combination with EasyOne Connect software for central data review and handling, as well as access to more advanced PFT software functionality. Features include:
- Powerful commenter functionality
- Encouraging pediatric incentives
- Custom provocation protocols
- Import external PFT data
For more information, please visit the EasyOne Connect product page.
Powerful EMR EHR Integration Engine
EasyOne Connect software includes powerful EMR EHR interoperability and IT management functionality for streamlined workflows. Features include:
- Proven EMR integrations
- Supports integration standards - HL7, XML, API, GDT, etc.
- Active directory integration
- SQL Server database
For more information, please visit the EMR EHR interoperability solutions page.
Direct Print Solution
EasyOne Air offers the unique option to print out test results directly without the need of a PC. A large variety of printers is supported and can be used without further installation. This makes the EasyOne Air one of the most mobile spirometry devices on the market.
- Print from a PC through EasyOne Connect or connect your printer directly via USB cable
- You can use EasyOne Air with both thermal printers and printers for ordinary paper
- Printers must support at least one of the following printer languages / formats: Direct PDF, Postscript, HP PCL 3 Enhanced protocol or HP PCL3GUI (Firmware ≥ V1.10.0)
Please check out the application note about printing with EasyOne Air.
Customized Reports
Customize reports and chose the parameters to be displayed.
- Choose graphs to be displayed
- Color reports for better visibility
- Arrange graphs and parameters through EasyOne Connect
- Choose between Z-score, % predicted and LLN through EasyOne Connect
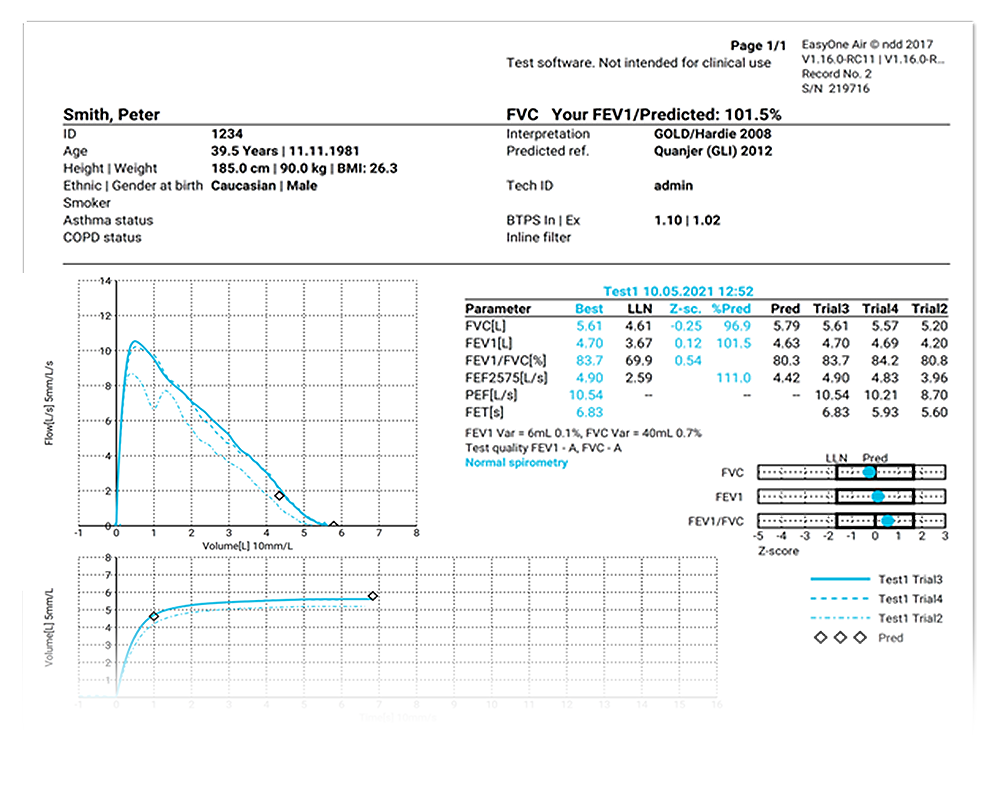
QC-Grading and Result Interpretation
Get automatic quality grading and interpretation based on NLHEP and ATS/ERS with the EasyOne Air.
- Quality grades based on NLHEP and ATS/ERS 2005 and 2019 for all tests
- ATS/ERS interpretation
- GOLD/Hardie, NICE, JRS and NLHEP
Cyber Security Protection for IT and Patient Information
Protect your patient information. EasyOne Air is a modern system designed to fulfill your cyber security needs.
- Encrypted database
- User authentication with automatic log off
- User permission levels
- FDA Approval in 2017 with cyber security documentation

Worldwide Compliance with Standards
Stay compliant with ATS/ERS standards and specific country regulations.
- Fully conformant to ATS/ERS 2005 and 2019 and ISO standards
- Worldwide approvals
- CE Approval
- FDA Approval
Click here for a complete list of ndd certificates.

TrueFlow™ - Trusted Ultrasound Technology
Stop worrying about calibration or accuracy of flow measurements. TrueFlow™ is the only ultrasound technology proven to be accurate for a lifetime for flow and volume measurements.
- Proven long-term stability
- Contact and resistance-free measurement
- Excellent accuracy and robustness
- Patented technology
To review case studies and additional information about our proven technology, please click here.
Large Selection of Predicted Sets (%Pred, Z-score and LLN)
The first in line for newly published predicted values: EasyOne devices are used in predicted studies worldwide (e.g. LUNOKID, ALAT).
- %Pred, Z-score, LLN
- GLI (Stanojevic 2009, Quanjer 2012)
- NHANES III (North America)
- PLATINO (South America)
- ERS (Europe) and JRS, IP, Chhabra (Asia)
Please see our specifications below for a complete list of the supported predicted values.
Infection Control
The EasyOne Filter solutions provide additional protection for those who wish to include a filter when performing tests.
- The EasyOne Filter keeps the ambient environment clean for technicians and patients.
- The ndd breathing mouthpiece protects the flow sensor from contamination.
- Fully passes 13 Waveform test as required by the 2019 ATS/ERS standard.
- Available for most testing environments where filters are required.
Avoid cross-contamination and keep cleaning to an absolute minimum:
- All parts exposed to the patient’s breath are single-patient-use.
- The sensor is protected from contamination by the ndd breathing mouthpiece.
- Only simple surface cleaning is required for the ndd device.
- No special storage conditions are required for single-patient use Spirettes and FlowTubes (ndd breathing mouthpieces).
Visit our Infection Control page for more information.
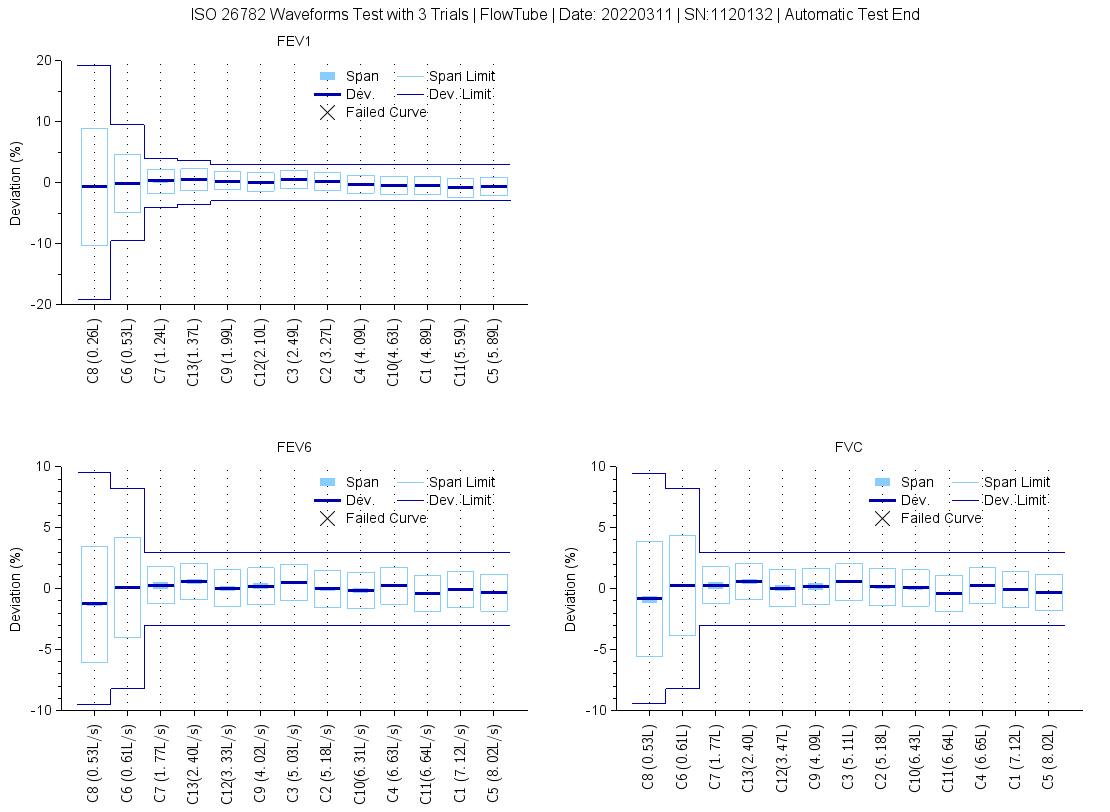
EasyOne Filter design allows for lung function testing accuracy and infection control. Fully passes ISO 13 waveform testing required by ATS/ERS (pictured above).
Downloads
 Software
Software

- EasyOne Connect V03.09.07.05Release date: 2025-07-17Related documents
- EasyOne Air Firmware V1.23.0Release date: 2025-09-01Related documents
- Updating the Software on EasyOne Air249.34 KB
- EasyOne Air Firmware Version History760.11 KB
Important: When updating your EasyOne Air device, please note the following: The EasyOne Connect software will update both your device and PC software. The EasyOne Air firmware update applies only to standalone devices.
 Application Notes
Application Notes

 Brochures
Brochures

 Certificates
Certificates

 Manuals
Manuals

FAQs
Which EHR vendors can ndd integrate with?
ndd can integrate with any EHR vendor that can support a bi-directional HL7 orders/results lab interface.
What kind of integration options does ndd offer?
ndd offers a file-based integration utilizing an SFTP or network-based location for file communication with an EHR vendor. HL7 is the standard data structure that ndd utilizes but can support other data formats as well. The use of other interface engines can be utilized to complete an integration project.
How long does the integration process typically take?
Duration depends on the IT team of the facility. The ndd side of the integration process can be completed within a few hours for standard integrations. EasyOne Connect software is simply configured to point to the SFTP or network-based location where files are being placed.
How does ndd ensure that data is securely transferred during integration?
EasyOne Connect software utilizes AES 256 encryption. SFTP has its own security protocols built into it. If a network-based location is utilized, it will follow the protocols of the facility.
Can patient data and test results be accessed across multiple laptops and desktops within a clinic?
Yes, EasyOne Connect offers an optional centralized patient database feature, enabling seamless access to patient orders and results across all instances of the software, irrespective of physical location. Customers can leverage their on-premise SQL servers or MS Azure environments to ensure data accessibility and consistency.
Does ndd offer user management within the software?
EasyOne Connect provides flexible options for managing users and their access. User accounts can be created and managed directly within EasyOne Connect for simple permission control. For more advanced management, the software supports integration with Active Directory for using your existing directory service accounts, groups, and permissions. EasyOne Connect also supports optional single sign-on (SSO) capability. If you would like to review the Active Directory Application Note, please contact [email protected].
Can ndd provide examples of successful integration with other clients?
ndd has integrated with all major vendors and can provide examples of successful integrations with various healthcare providers and organizations. You can contact our integration department via email at [email protected] for more information.
How does ndd handle updates and maintenance to the integration?
ndd does not handle the maintenance and upgrades as the software is installed locally on the client’s machine. We do, however, offer a silent installer package that our support team can assist you with. This silent installer can be executed via a script through active directory commands depending on your facility. It is the responsibility of the facility to implement any future updates. There is no licensing fee associated with the software and/or the updates.
Are there any additional costs associated with integration?
ndd does not charge for standard integration. In the event custom development is needed, ndd will provide a statement of work before a project begins. Almost all EHR vendors will charge a fee that the client is responsible for.
What are the HL7 specifications?
HL7 specifications can be found here.
What are the database options?
- Local SQLite file-based database.
- On-premise Microsoft SQL Server database.
- Azure cloud-hosted Microsoft SQL Server database.
Please see the application note for additional information.
Who is responsible for setting up and hosting the SFTP and/or network-based location?
The facility is responsible for setting up and hosting either option. ndd requires a username and password if using an SFTP environment.
What results are received in the HL7 message?
The result HL7 message will consist of a combination of discrete results and an embedded base64 PDF attachment for the PFT report. If you prefer, we also offer the option of including a reference link instead of the embedded PDF.
Is a server required?
No, a server is not required.
What kind of support does ndd offer during and after the integration process?
You can schedule a meeting with an integration engineer from 8 a.m. to 5 p.m. ET Monday through Friday. ndd can accommodate meetings outside of that timeframe if planned accordingly. The integration team can be reached via email at [email protected]. The support team can also be reached via email at [email protected] or by phone at (978) 470-0923.
Which predicted or normal values are supported?
You can find the product specification on the respective product pages. The specification contains a list of all supported normal values.
To get more detailed information about the supported parameters, age range, ethnicities and height range of each supported predicted set, please click here.
What is BTPS and how is it used?
BTPS Correction is used to convert flow and volume measured at ambient conditions to the conditions within the lungs. Ambient conditions are called ATP (ambient temperature, pressure); the conditions within the lungs are called BTPS (body temperature, pressure, water vapor saturated).
Click here for more information.
Where can I purchase the EasyOne Air spirometer?
All our products can be purchased through our authorized distribution partners. Please contact us to receive more information on finding your local dealer.
What are my options for printing results from the EasyOne Air?
A compatible printer can be connected directly to the cradle provided with the device. Please consult our Printer Compatibility List including thermal printer options.
You can also use our free companion PC software, EasyOne Connect. The device is connected to the PC via the USB cradle provided. Data from the spirometer is synchronized with the PC database, allowing you to print to any printer connected to that PC or network. EasyOne Connect also allows you to view, trend and create PDF reports.
It is also possible to send pdf reports directly to a connected PC via file exchange.
What consumables are required for the EasyOne Air spirometer?
The EasyOne Air uses the patented ndd Flow Tube, a single-patient-use mouthpiece which ensures that no contamination can reach the internal flow path of the device. This means that no cleaning is required between patients, and no calibration, ever. Simply toss the Flow Tube after each test session.
What are the options and benefits of connecting the device to my PC?
EasyOne Connect is the free companion PC software for the EasyOne Air. It can be downloaded directly from our web site. Here are a few of the features and benefits of using EasyOne Connect:
- Long-term storage of test data
- Ability to enter patient demographics from the PC
- Connectivity with your EMR system
- Ability to over-read and manipulate data on the PC
- Export of data in many different formats, including the ability to create PDF reports
- Bluetooth allows wireless viewing of real-time curves on PC display, or use of real-time animated/pediatric incentives
- View and print trend graphs
- Create custom test report templates
How many tests can be saved in the device's memory?
The on-board memory for the EasyOne Air spirometer can store approximately 10,000 tests. For long-term storage the data can be synchronized to the EasyOne Connect PC software database.
How long does the battery last?
The EasyOne Air spirometer uses a rechargeable lithium-ion battery. An empty battery can be fully charged in about 5 hours. A fully-charged battery can support the performance of approximately 100 spirometry test sessions. While the battery life is highly dependent on the intensity of use, ndd generally recommends that the battery be replaced every 3 years to ensure the best user experience.
Do I need to calibrate the EasyOne Air spirometer?
No. All EasyOne products are designed to not require calibration. ndd’s patented TrueFlow technology is the only technology proven to remain stable over its lifetime. A number of independent papers have been published that confirm this long-term stability.
For organizations like NIOSH/OSHA or Social Security/Disability, where calibration checks are required, ndd offers a 3-Liter calibration syringe. A calibration check (cal-check) is not to be confused with calibration. A cal-check simply validates that the device is within calibration limits. Unlike calibration, a cal-check does not adjust the calibration values.
How can I change the ATS/ERS Spiromety Standard setting on my EasyOne Air device
The ATS/ERS spirometry standard setting can be viewed or changed in the menu: Tools > Settings > Spirometry.
The setting is called ATS/ERS Standard. Changes will only affect future tests. Existing tests will not be affected.
How long does a spirometry test take?
A spirometry test typically takes 10 to 15 minutes, depending on the patient’s ability to follow instructions and complete the required breathing maneuvers. The test involves taking a deep breath and forcefully exhaling into a spirometry device multiple times to achieve accurate results.
Healthcare providers may repeat the test after administering a bronchodilator to assess lung function improvement. Proper coaching and technique are essential for reliable measurements.
Learn how to perform a spirometry test step by step.
Read the blog.
How to backup my database in EasyOne Connect
In EasyOne Connect, go to: Utilities > Export Data > tick Patient Data > click Export. Save the file in the preferred location.
Learn more by watching the video below.
How to backup the database from an EasyOne Air
In this instruction will guide you through the options to backup a database from the EasyOne Air device. Prepare your device and have an SD card ready to follow along with the instructions. Let’s have a look on how to backup from the Easy One Air.
- To perform this operation you need an SD card.
- The SD card slot is located next to the battery. To access it, open the battery door and remove the battery.
Alternative to the SD card database backup you can also synchronize the tests to your EasyOne Connect on the PC and save the database from there, but be aware that existing tests will be merged. We will not discuss this option here, but focus on the option when using the stand alone device. Check the EasyOne Connect training module for a more comprehensive instruction to backup on the PC software.
- Ensure the SD card is inserted and turn on the device.
- In the menu, go to Tools > Database > Backup and confirm with Yes.
- Enter your administrator password and confirm with OK. Optionally enter an encryption password. Important! If a password is entered here, it will need to be entered in the restore steps. This password cannot be recovered.
- Click confirm and then again on OK
All set. The database is saved on your SD card.
In this instruction will guide you through the options to backup a database from the EasyOne Air device. Prepare your device and have an SD card ready to follow along with the instructions. Let’s have a look on how to backup from the EasyOne Air.
- To perform this operation you need an SD card.
- The SD card slot is located next to the battery. To access it, open the battery door and remove the battery.
Alternative to the SD card database backup you can also synchronize the tests to your EasyOne Connect on the PC and save the database from there, but be aware that existing tests will be merged. We will not discuss this option here, but focus on the option when using the stand alone device. Check the EasyOne Connect training module for a more comprehensive instruction to backup on the PC software.
- Ensure the SD card is inserted and turn on the device.
- In the menu, go to Tools > Database > Backup and confirm with Yes.
- Enter your administrator password (default 8005) and confirm with OK. Optionally enter an encryption password. Important! If a password is entered here, it will need to be entered in the restore steps. This password cannot be recovered.
- Click confirm and then again on OK
All set. The database is saved on your SD card.
Learn more by watching the video below.
For more information see the AppNote.
How to change the header information on my EasyOne Air device
The EasyOne Air header information can be changed in Tools > Settings > Print.
The header information will appear on reports directly printed from the device.
Learn more by watching the video below.
How to check which firmware version is installed on my EasyOne Air device
The EasyOne Air firmware version can be found in Tools > Device.
Learn more by watching the video below.
How to coach a patient to perform spirometry
The typical spirometry exam is a three-phased process – each with their own coaching techniques to help patients produce an effective test.
- Phase 1
In the first phase, the goal is to instruct the patient on the proper techniques to apply their mouth to the spirometry mouthpiece. Best results are achieved when the patient takes a deep, deep breath, placing the mouthpiece between their teeth, and sealing the mouthpiece with their lips. - Phase 2
The second phase is the BLAST. This is when the patient will blow out as hard and as fast as possible. Coaching a patient in this phase is best achieved by developing a rapport with them prior to the exam, as the tester will learn which level of coaching is best for that individual patient. - Phase 3
The final phase is the remaining 5 to 6 seconds of exhalation. In this phase, you’ll quietly encourage the patient to “keep blowing” to complete the exhalation.
- Phase 1
How to create a new patient database in EasyOne Connect
A new database can be created in Utilities > Configuration > General tab > Storage tab, click New, Select File Based, click New Name the file, click Save.
Setting a password is optional. This password is needed to open the database and cannot be recovered. Make sure to remember the password, otherwise the data cannot be accessed.
Click OK, Click save.
The software will refresh and the new database will be loaded.
Learn more by watching the video below.
How to enable User Logon on my EasyOne Air device
User handling can be enabled to define certain user permission levels and require a log on when the device is turned ON.
- Users can be set up in Tools > User.
- Enter the admin password to access the settings.
- Select ‘Add User’ to create a new user. Users are assigned to a user group.
- There needs to be at least one administrator who has access to all menus and functions. Technicians have restricted access.
- Confirm the information entered is correct.
Enable user logon. Go to: Tools > Settings > Security.
- Enter the default admin password (default 8005)
- Select ‘User logon required’
The device will then prompt a logon when it is turned ON.
Learn more by watching the video below.
How to export a single patient database
ndd Service and Support may request a single patient database for review if there are any questions about test performance.
A single patient database can be exported from EasyOne Connect in the following:
Patients > Select the patient > Click the double arrows to expand the menu > Select Database > Click Save File as and save the file > Click Export.
How to export the XML database from EasyOne Connect and convert it to a CSV file
The EasyOne Connect Patient Data can be exported as an XML file and converted to a CSV file. First locate the EasyOne Connect Data Export application note found on our website.
- Click on the link in the app note to download the zip file, which contains a library of required files needed for the conversion.
- Extract the files from the ZIP folder.
- In Easy One Connect export the XML file of the database. Go to: Utilities > export XML.
- Save the XML file in the XML input folder in the conversion file library.
This will export the entire database. Single tests can also be exported as an XML file in the patient history.
The Conversion File library contains several conversion options that include different parameters.
- Double click on a bat file to run the conversion. This will convert any files in the XML input folder.
Press any key to close out of the conversion window.
The converted CSV file is found in the CSV output folder.
Learn more by watching the video below.
How to find the logfile on the EasyOne Air
Follow these steps to find the EasyOne Air log file.
First connect the cradle to the PC and then dock the EasyOne Air device.
Next, open the File Explorer, find this PC, EasyOne Air and exchange.
The log file will be named syslog.txt
How to import a patient database
A database can be imported into the currently selected database in Utilities Configuration, the General tab, the Storage tab, click Import, Select File Based.
Click select. Select the database file to be opened.
Click Open. Click OK.
When the import is complete, click Save.
The chosen database will be merged with the currently selected database.
Learn more by watching the video below.
How to locate the currently selected database in EasyOne Connect
In EasyOne Connect, go to: Utilities > Configuration > General (tab) > Storage (tab) and there it is listed under database name. Default location on the PC:
C:\ProgramData\ndd\EasyOne Connect\EasyOneConnect.sqlite.
Learn more by watching the video below.
How to restore a database backup on the EasyOne Air device
This instruction will guide you through the options to restore a database backup on the EasyOne Air device. The backup data is located within the backup folder on the SD card. See how to perform a backup. If the backup data is encrypted, the encryption password is needed to perform the database restore.
All data created and settings change during the time between backup and restore will be permanently lost, which also includes audit trail entries.
It is strongly recommended to perform a backup before restoring the database.
A database restore can only be performed by users with admin level rights or by the default admin user if user management is not activated. Let’s have a look on how to restore a database to the EasyOne Air.
- To perform this operation, you need an SD card with a backup on it. The SD card slot is located next to the battery. To access it, open the battery door and remove the battery.
- In the menu, go to: Tools > Database > Restore and confirm with Yes.
- Enter your administrator password.
- Confirm with OK.
- Now select the backup database you would like to restore. Optionally enter an encryption password. If the file is not password protected, then leave the password field empty and click Confirm.
- Here you go, the database is being restored. Wait for the operation to be completed and click OK.
The device will request a reboot. Follow along with the instructions on the screen and check the results in your restored database.
Learn more by watching the video below.
For more information see the AppNote.
How to select a different patient database in EasyOne Connect
A different database can be selected in Utilities > Configuration > General tab > Storage tab.
Click Select > Select File Based.
Click Select. Select the database file to be opened. Click Open Review the database path. Click OK Click Save.
The software will refresh.
Learn more by watching the video below.
How to select the predicted reference and interpretation settings on my EasyOne Air device
The selected predicted reference and interpretation can be changed in the menu: Tools > Settings > Spirometry.
Refer to the Predicted Reference AppNote for more information on each of the options.
Predicted Set 2 is for pediatric selections.
The interpretation can be changed under System Interpretation.
Previous tests can be updated with the ‘Recalculate’ button under Patients or in the EasyOne Connect software.
If a test is started in EasyOne Connect via the Bluetooth feature, EasyOne Connect settings will be used.
Changing the settings on the EasyOne Air device only applies for standalone tests.
Learn more by watching the video below.
For more information see the App Notes Predicted Normal Values Overview and Recommended Printers for EasyOne Pro.
How to troubleshoot connection issues (printing or synchronization) on the EasyOne Air device or cradle
A possible issue with the EasyOne Air device is USB connection issue. These prevent data from synchronizing to the PC or printing in a direct print setup. Here are some steps to try to troubleshoot the issue.
- Ensure the USB cable is connected to the correct port on the cradle. The USB cable should be inserted in the port on the left of the back of the cradle.
- USB connection issues can be caused by damaged cables or ports. Check the USB cable to ensure there is no damage to the cable or connector.
- Try a different USB port on the PC or a different PC.
- Do not use a USB hub.
If these troubleshooting steps do not resolve the issue, contact local IT to check for any USB port blocks or PC settings that could prevent connection.

Learn more by watching the video below.
How to troubleshoot FlowTube detection or insertion issues on the EasyOne Air device
Each time a test is started, the EasyOne Air will recognize the correct position of the FlowTube and provide feedback. If the FlowTube is not placed correctly, the device feedback may include:
- No EasyOne FlowTube detected
- Please use an EasyOne FlowTube
- Check the EasyOne FlowTube for damage
- Check for correct EasyOne FlowTube insertion
A troubleshooting step for the FlowTube detection error is to try a new FlowTube as a damaged one can cause this error.
Another troubleshooting step is to ensure the FlowTube is fully inserted into the device, align the arrows and push the FlowTube in until the FlowTube is flush against the device.
The FlowTube detection error could also result from residue in the sensor tube.
Cleaning inside the sensor tube can cause these residues and should be avoided.
Please refer to the operator’s manual for cleaning instructions.
Learn more by watching the video below.
How to update the EasyOne Air firmware via an SD card (only with instructions from ndd service and support)
The installer file is provided by the ndd service and support team on request. The purpose of the SD card update is to execute the update directly and force it as soon as the device is turned on. Minimal user interaction is needed other than inserting and removing the SD card.
First, let’s prepare our card. These steps need to be done on your PC.
- Grab the ZIP file you received from ndd service and support and copy it to the SD card.
- Unzip it there.
- Then open the folder and mark the three files.
- Copy them to the top level folder of the SD card. The card is now ready.
Label this SD card accordingly with the firmware version or ensure to delete the files after our update is completed.
- Safely remove the SD card from the PC and let’s use it on the EasyOne Air device.
- Unlock the battery door with an appropriate tool, turn the door lock clockwise to unlock, then remove the battery.
- Insert the SD card, insert the battery and close the battery door again.
- Use an appropriate tool to lock it.
- Now turn on the device, read the information on the screen and confirm you’d like to update to the mentioned firmware version. The update should start right away.
- Do not interrupt the update process. Once the progress bar is full, follow the instructions on the screen, confirm with OK, and let the device turn off.
You have updated your EasyOne Air device successfully.
Don’t forget to remove the SD card before you start using it for testing.
Learn more by watching the video below.
For more information see the AppNote.
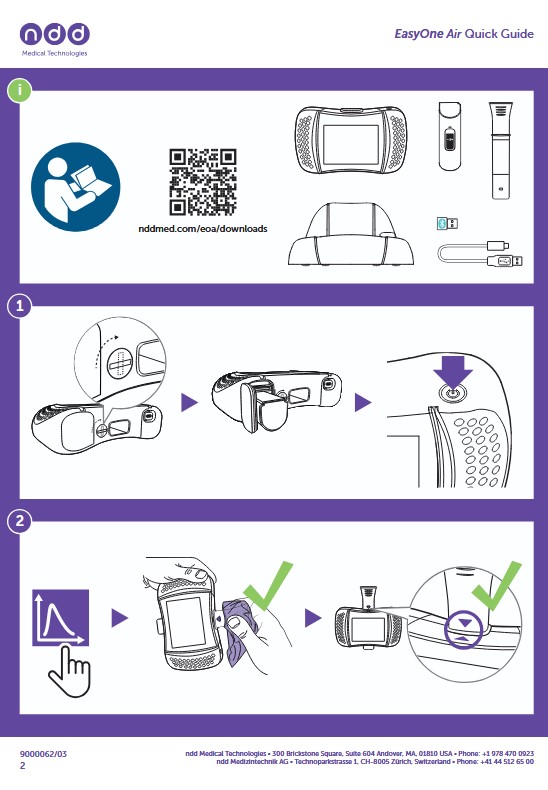
How to update the EasyOne Air firmware via EasyOne Connect software or exchange drive
This instruction will guide you through the options to update the firmware on your EasyOne Air device. Prepare your device to follow along with the instructions.
There are mainly three ways:
- via the EasyOne Connect software
- via the Exchange Drive
- via an SD card
This instruction will guide you step by step through the procedure of option 1. and 2. We will handle the SD card update (option 3) in a separate FAQ.
Software updating via EasyOne Connect is the preferred updating method. If you’re using the device in combination with multiple EasyOne Connect PCs, it may be required that also the other PCs need to be updated. Please refer to the version history for software compatibility, which is available on the ndd website.
- To start the procedure, download the latest EasyOne Connect software from the ndd website and run the installer.
- Connect the EasyOne Air USB cradle to the PC.
- Start the EasyOne Connect software, turn on the EasyOne Air and place it into the USB cradle
- You will see a message indicating a software mismatch and an option to update the device. Choose yes.
- The firmware is now prepared for the update and shortly you will see a progress bar indicating the status.
- Leave the device connected until the update process is completed.
Let’s continue with the update via the Exchange drive. (Option 2) If you are not using the device in combination with the EasyOne Connect software, you will need to go through this procedure.
- To start the procedure, download the latest EasyOne Air firmware from the ndd website.
- Connect the EasyOne Air to the PC via the cradle.
- Turn it ON and the device will appear on your PC as a drive.
- Open it and you will see the Exchange drive.
- Copy the downloaded firmware file to it.
- Now that the firmware is stored on the device, we can simply perform the update from the device menu.
- To start the update, take the EasyOne Air off the cradle and go to Tools > Update.
- The device invites you to install the downloaded firmware file.
- Click OK
- If requested, enter the user password.
- Wait a few minutes for the update to complete. Do not turn off the device until the update has finished.
- Once the update is complete, choose OK to shut down the device.
Learn more by watching the video below.
For more information see the AppNote.
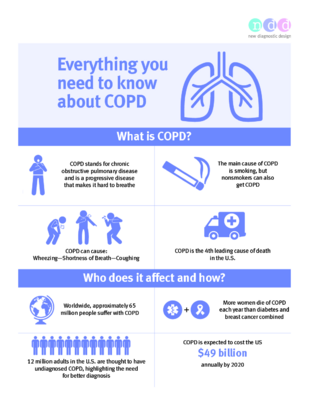
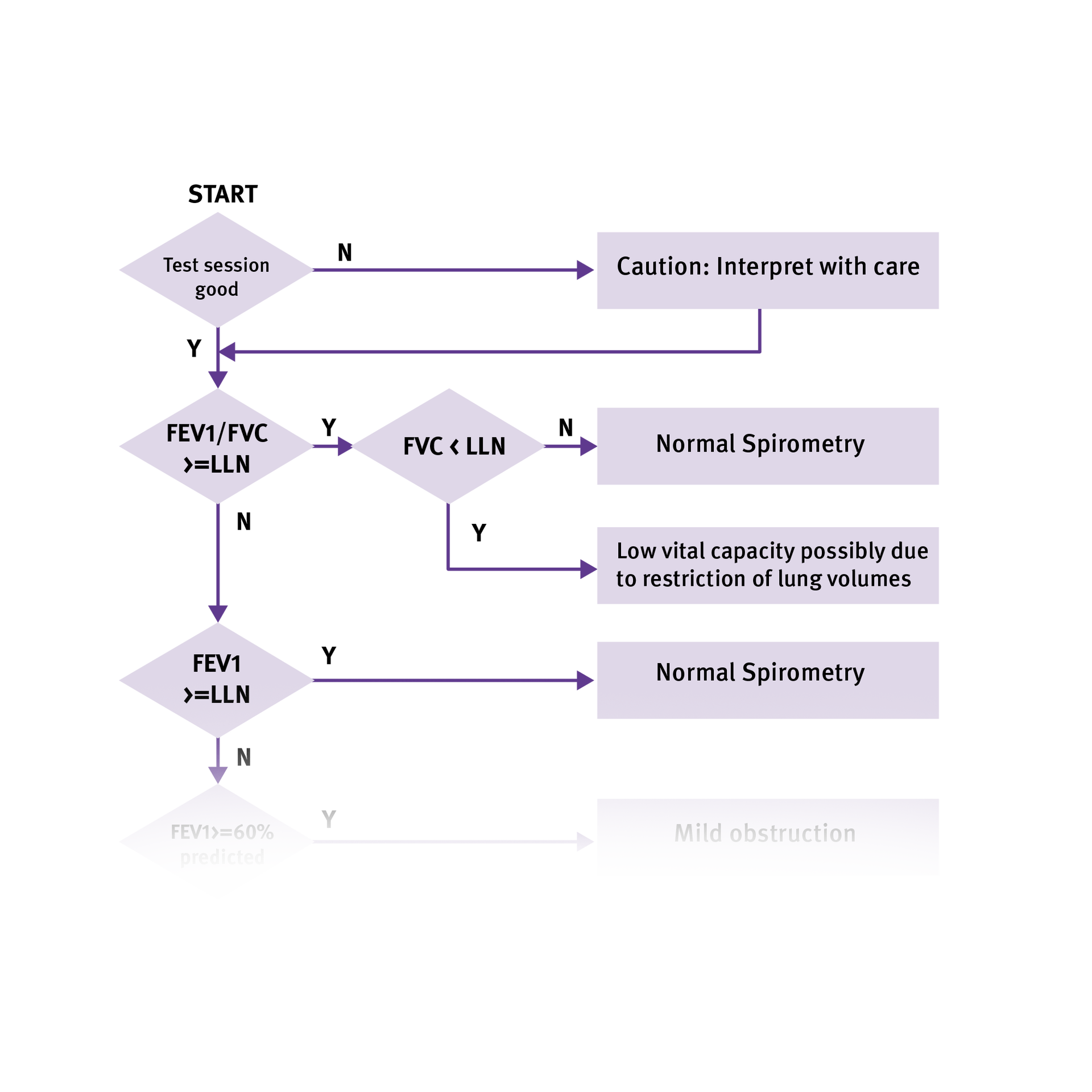
What are examples of obstructive lung diseases?
A few examples of obstructive lung disease include:
- Chronic Obstructive Pulmonary Disease (COPD)
- Emphysema
- Chronic Bronchitis
- Asthma
- Bronchiectasis
What are examples of restrictive lung disease?
A restrictive lung disease is exactly that – one where a restriction occurs in the respiratory system.
A few examples of restrictive lung disease include:
- Interstitial lung diseases such as idiopathic pulmonary fibrosis
- Sarcoidosis
- Obesity
- Scoliosis
- Neuromuscular diseases such as muscular dystrophy or ALS
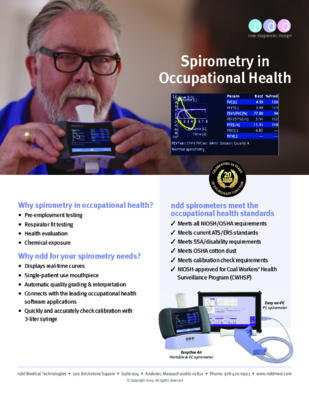
What are the important parameters in spirometry?
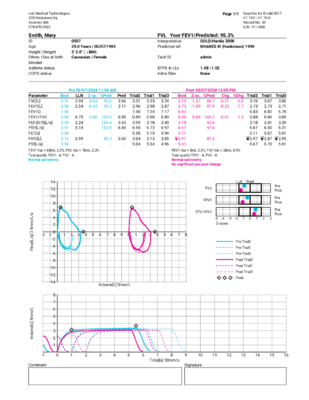
A spirometry test will produce a series of results or parameters that help medical professionals easily complete this exam. The three most important spirometry parameters are the FEV1, FVC, and FEV1/FVC ratio parameters.
A simple guide to reading spirometry results.
Get the tips from Buddy
What indictations are used for spirometry?
There are multiple patient-symptoms or medical conditions that justify the use of spirometry. A few of the most common indicators include:
- To manage asthma and COPD patients
- Evaluate shortness of breath
- Perform surveillance for occupational-related lung disease
- Evaluate former or current smokers over the age of 45 for COPD
- Classification of COPD. (COPD is an umbrella term that covers multiple respiratory diseases including chronic bronchitis, emphysema, and idiopathic pulmonary fibrosis (IPF))
- Monitor disease progression
- Measure response and progress due to treatment, medication, and respiratory therapy
- Smoking cessation
Tips from leading experts on the value of spirometry.
Watch the webinar
What is a diagnostic spirometer?
A diagnostic spirometer is a medical device that is used to measure lung function. It can be a portable or stationary spirometry device that is operated by a medical professional who is specially trained in proper testing procedures and techniques to produce a more consistent and reliable result.
ndd EasyOne spirometry solutions
Speak to a clinical specialist today!
###
What is FEV1 in spirometry and what does it tell you?
FEV stands for Forced Expiratory Volume. The FEV1 parameter equates to the volume that is exhaled after one second. It is intended to measure the severity of an obstruction – with average FEV1 results measuring two to four liters. A lower FEV1 result equates to higher obstruction.
A simple guide to reading spirometry test results.
Get the tips from Buddy
What is FVC in spirometry and what does it tell you?
FVC stands for Forced Vital Capacity. FVC determines the total exhaled volume of air. FVC tells the spirometry tester how much air volume the patient can exhale. The FVC will fall when the patient can’t inhale deeply or can’t exhale completely. The average FVC result for an elderly man is about four liters (one gallon) of air. The intent of this parameter is to detect restriction.
A simple guide to reading spirometry test results.
Get the tips from Buddy.
What is spirometry used for?
Spirometry is used to diagnose and monitor lung diseases, assess pulmonary function, and support occupational health screenings. Spirometry helps healthcare providers to:
- Diagnose respiratory conditions like COPD and asthma
- Monitor disease progression and treatment effectiveness in patients with chronic lung conditions
- Evaluate lung function in workplace health programs, particularly for individuals exposed to dust, chemicals, or other respiratory hazards
By using a diagnostic spirometer, medical professionals can detect lung diseases early, track changes over time, and ensure workplace safety.
Ready to add spirometry testing to your practice?
Discover more now.
What is spirometry?
Spirometry is the primary method of assessing overall lung function. It works by measuring the volume of air that a patient can forcefully expel from the lungs after inhaling as much as possible. This non-invasive test is completed by medical professionals and is essential for diagnosing and monitoring respiratory conditions like COPD, asthma, and other lung diseases.
Using a diagnostic spirometer, a trained healthcare professional can assess lung health, detect early signs of disease, and guide treatment decisions.
Discover our advanced spirometry solutions here.
Book a demo today!
What is the difference between spirometry and a pulmonary function test (PFT)?
A spirometry test is a specific type of pulmonary function test. Spirometry measures the lungs volume (or how much) and flow (how quickly) the patient can move air into and out of their lungs. Spirometry will tell the tester if the patient has an obstruction, restriction, a mixed defect, or has normal lung flow.
Spirometry does NOT look at gas exchange and does not provide absolute lung volumes (RV, FRC, and TLC).
Diffusion capacity or transfer factor of the lung for carbon monoxide (CO) is known as a DLCO test. This measures the gas exchange and is used in conjunction with spirometry to provide a differential diagnosis. DLCO is also used to assess disease severity and is one of the best correlates of emphysema in COPD.
The final component that completes the full PFT exam is measuring absolute lung volume (RV, FRC, and TLC). This is completed by measuring body plethysmography, gas dilution, or nitrogen washout. Lung volumes are commonly used for the diagnosis of restriction. In obstructive lung disease, they are used to assess for hyperinflation. The changes in lung volumes can also be seen in a number of other clinical conditions.
Want to learn more about adding PFT and spirometry to your practice?
Get a live demo today!
What is the FEV1/FVC Ratio in spirometry and what does it tell you?
The FEV1/FVC ratio is typically expressed as a percentage (such as 75%). Nearly three-fourths of lung volume can normally be exhaled during the first second of the spirometry test. As such, the normal ratio falls between 65 to 85 percent. The range of normal FEV1/FVC ratio is determined by the patient’s age.
A simple guide to reading spirometry test results.
Get the tips from Buddy.
Which print options do I have with the EasyOne Air device
You can also synchronize the test to your EasyOne Connect on the PC and print from there.
We will not discuss this option here, we will focus on the options when using the standalone device
The print options need to be selected in the EasyOne Air menu first. This is how to do it.
- From the main menu, go to Tools > Settings > Print and select the Print format. In this view you can set up all printer related settings.
Let’s start with the most common option, which is external printing (Option 1). This involves a direct connection from the cradle to a printer.
- Ensure to connect the micro USB end of cable to the correct port on the cradle and connect the USB type B end to the printer.
- There is a large choice of printers on the market, each supporting different print formats or languages. In order to print successfully, the printer must support at least one of these formats. Direct PDF, HP PCL 3 Enhanced protocol, HP PCL3GUI
- A compatible printer list can be found on the ndd website.
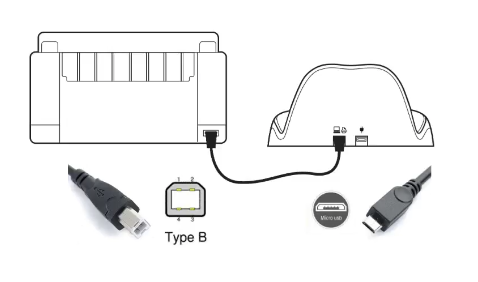
Another popular way to print results is via the integrated PDF printer (Option 2). This will create a PDF file in the EasyOne Air storage so that it can be moved to your PC and further utilized.
Whenever you print a test result, the file will be stored in the Exchange drive.
- To access this file, connect the device to a PC via the USB cradle. Usually a pop up window opens displaying the Exchange drive. If no pop up window appears, try opening the Exchange drive manually. For example, navigate in the Windows Explorer to this PC, then select EasyOne Air, then Exchange and select the desired PDF print out.
- The Exchange drive is the EasyOne Air internal storage and has only 15 MB of storage space. In there you find all PDF prints and also the log file.
The third method is to print the results via thermal printer (Option 3).
This method requires a special thermal printer that is compatible with the EasyOne Air.
Once the printer is connected, the results can be printed out immediately.
Learn more by watching the video below.
For more information see the AppNote.
Specifications
Standards & Recommendations
Quality, Medical Devices & ElectricalIEC 60601-1, IEC 60601-1-2, IEC 62304, IEC 62366, ISO 13485, ISO 14971, ISO 26782, ISO 23747FDA510(k) market clearanceAssociations & InstitutesATS/ERS 2005 spirometry standard, ATS/ERS 2019 spirometry standard, ATS/ERS 2022 interpretation strategies, NIOSH, OSHA, SSA DisabilityLanguages
Available languagesEnglish, Danish, Dutch, French, German, Italian, Norwegian, Polish, Portuguese, Russian, Spanish, SwedishTechnical
Printing optionsDirect to printer or with EasyOne Connect softwareData managementEasyOne Connect (SQLite, MS SQL Server)ExportHL7, XML, GDT, with EasyOne Connect softwareData linksUSB, BluetoothTest storage>10,000 testsAge rangeSpirometry ≥4 yearsDimensions87 x 155 x 36 mm (H x W x D), 356 g,
3.4 x 6.1 x 1.4‘‘ (H x W x D), 13 ozDevice classificationType BF applied partOperating conditionsTemp 0 - 40 °C/ 32 - 104 °F, Rel. Humidity 5 - 90%, Atmosph. Pressure 700 - 1060 hPaPower supplyRechargeable lithum-ion battery, USB power supplyRechargeable batteryExchangeable, 3.6 VDCParameters
FVCATI, BEV, EOTV, FEF10, FEF25, FEF25-75, FEF25-75_6, FEF40, FEF50, FEF50/FVC, FEF50/VCmax, FEF60, FEF75, FEF75-85, FEF80, FET, FET25-75, FEV.25, FEV.5, FEV.5/FVC, FEV.75, FEV.75/FEV6, FEV.75/FVC, FEV.75/VCmax, FEV1, FEV1/FEV6, FEV1/FVC, FEV1/FVC6, FEV1/VC, FEV1/VCmax, FEV1Q, FEV3/FVC, FEV3/VCmax, FEV3, FEV6, FVC, MEF20, MEF25, MEF40, MEF50, MEF60, MEF75, MEF90, MMEF, MTC1, MTC2, MTC3, MTCR, PEF, PEFT, t0, VC, VcmaxFVLATI, BEV, CVI, E50/I50, EOTV, FEF10, FEF25, FEF25-75, FEF25-75_6, FEF40, FEF50, FEF50/FVC, FEF50/VCmax, FEF60, FEF75, FEF75-85, FEF80, FET, FET25-75, FEV.25, FEV.5, FEV.5/FVC, FEV.75, FEV.75/FEV6, FEV.75/FVC, FEV.75/VCmax, FEV1, FEV1/FEV6, FEV1/FIV1, FEV1/FIVC, FEV1/FVC, FEV1/VC, FEV1/VCmax, FEV3/FVC, FEV3/VCmax, FEV1Q, FEV3, FEV6, FIF25, FIF 25-75, FIF50, FIF50/FEF50, FIF75, FIV.25, FIV.5, FIV1, FIVC, FVC, MEF20, MEF25, MEF40, MEF50, MEF60, MEF75, MEF90, MIF25, MIF50, MIF75, MMEF, MMIF, MTC1, MTC2, MTC3, MTCR, PEF, PEFT, PIF, t0, VC, VCmaxSVCERV, IC, IRV, Rf, VC, VCex, VCin, VCmax, VTMVVMVV, MVV6, MVVtime, Rf, VCext, VTPredicted normal values Spirometry
GLIStanojevic 2009, Quanjer 2012, Bowerman 2023 (Global GLI)North AmericaNHANES III (Hankinson) 1999, Knudson 1983, Knudson 1976, Crapo 1981, Morris 1971 & 1976, Hsu 1979, Dockery (Harvard) 1993, Dockery (Harvard) 1993, Polgar 1971, Gutierrez (Canada) 2004, Eigen 2001, Cherniak 1972Latin AmericaChile 2010, Chile (Pediatrics) 1997, Pereira 1992, Pereira 2006/2008, Pérez-Padilla (PLATINO) 2006, Pérez-Padilla (Mexico) 2001, Pérez-Padilla (Mexico, Pediatrics) 2003EuropeERS (ECCS, EGKS, Quanjer) 1993, Garcia-Rio (SEPAR) 2013, Falaschetti 2004, Forche (Austria) 1988 & 1994, Klement (Russia) 1986, Roca (Spain, SEPAR) 1982, Rosenthal 1993, Sapaldia (Switzerland) 1996, Vilozni 2005, Zapletal 1977, Zapletal 2003Europe ScandinaviaHedenström (Sweden) 1985/1986, Gulsvik (Norway) 1985, Berglund Birath (Sweden) 1963, Langhammer (Norway) 2001, Finnish 1982/1998, Nystad 2002, Koillinen 1998, 2001, Kainu (Finland) 2016AustraliaHibbert 1989, Gore Crockett 1995AsiaChhabra (India) 2014, Dejsomritrutai (Thailand) 2000, (Indonesia) 1992, IP (China, HongKong) 2000 & 2006, JRS 2001 & 2014AfricaMengesha (Ethiopia), 1985Flow/Volume Sensor
Measurement PrincipleUltrasonic transit timeMeasuring range± 16 l/sFlow Resolution4 ml/sFlow Accuracy (except PEF)± 2% or 0.020 l/sPEF Accuracy± 5% or 0.200 l/sVolume Accuracy± 2% or 0.050 lMVV Accuracy± 5% or 5 l/minResistance<1.5 cm H20/l/s at 14 l/sOrdering Information Devices
Part NumbersDescription2500-2EasyOne AirOrdering Information Accessories
Part NumbersDescription5050-50EasyOne FlowTube, individually wrapped, box of 50 pcs5050-200EasyOne FlowTube, individually wrapped, box of 200 pcs5050-500EasyOne FlowTube, individually wrapped, box of 500 pcs (not available in all countries)2030-2ndd Calibration syringe 3L with EasyOne FlowTube Cal Check Adapter2500-50.1EasyOne Air USB cable B-micro (cradle to printer)2500-50.5EasyOne Air power supply with adapters
Accessories

EasyOne Filter
It’s not just a filter. It’s the EasyOne Filter.